PLoS Pathogens ( IF 6.7 ) Pub Date : 2020-03-16 , DOI: 10.1371/journal.ppat.1008394 Biao Zhou 1 , Xiangkai Zhen 1, 2 , Huan Zhou 3 , Feiyang Zhao 4 , Chenpeng Fan 5 , Vanja Perčulija 1 , Yigang Tong 4 , Zhiqiang Mi 6 , Songying Ouyang 1, 2
|
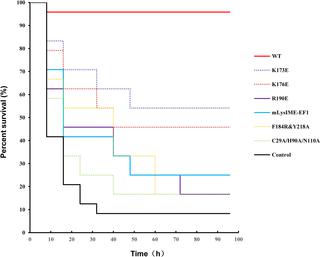
Using bacteriophage-derived endolysins as an alternative strategy for fighting drug-resistant bacteria has recently been garnering renewed interest. However, their application is still hindered by their narrow spectra of activity. In our previous work, we demonstrated that the endolysin LysIME-EF1 possesses efficient bactericidal activity against multiple strains of Enterococcus faecalis (E. faecalis). Herein, we observed an 8 kDa fragment and hypothesized that it contributes to LysIME-EF1 lytic activity. To examine our hypothesis, we determined the structure of LysIME-EF1 at 1.75 Å resolution. LysIME-EF1 exhibits a unique architecture in which one full-length LysIME-EF1 forms a tetramer with three additional C-terminal cell-wall binding domains (CBDs) that correspond to the abovementioned 8 kDa fragment. Furthermore, we identified an internal ribosomal binding site (RBS) and alternative start codon within LysIME-EF1 gene, which are demonstrated to be responsible for the translation of the truncated CBD. To elucidate the molecular mechanism for the lytic activity of LysIME-EF1, we combined mutagenesis, lytic activity assays and in vivo animal infection experiments. The results confirmed that the additional LysIME-EF1 CBDs are important for LysIME-EF1 architecture and its lytic activity. To our knowledge, this is the first determined structure of multimeric endolysin encoded by a single gene in E. faecalis phages. As such, it may provide valuable insights into designing potent endolysins against the opportunistic pathogen E. faecalis.
中文翻译:

粪肠球菌噬菌体中由单个基因编码的新型双组分细胞内溶素的结构和功能洞察。
使用噬菌体衍生的细胞内溶素作为对抗耐药细菌的替代策略最近重新引起了人们的兴趣。然而,它们的应用仍然受到它们狭窄的活性谱的阻碍。在我们之前的工作中,我们证明了细胞内溶素 LysIME-EF1 对多种粪肠球菌( E. faecalis )菌株具有有效的杀菌活性). 在此,我们观察到一个 8 kDa 片段并假设它有助于 LysIME-EF1 裂解活性。为了验证我们的假设,我们以 1.75 Å 的分辨率确定了 LysIME-EF1 的结构。LysIME-EF1 展示了一种独特的结构,其中一个全长 LysIME-EF1 形成一个四聚体,具有三个额外的 C 末端细胞壁结合结构域 (CBD),对应于上述 8 kDa 片段。此外,我们在LysIME-EF1基因中发现了一个内部核糖体结合位点 (RBS) 和替代起始密码子,它们被证明与截短的 CBD 的翻译有关。为了阐明 LysIME-EF1 裂解活性的分子机制,我们结合了诱变、裂解活性测定和体内动物感染实验。结果证实,额外的 LysIME-EF1 CBD 对 LysIME-EF1 结构及其裂解活性很重要。据我们所知,这是第一个确定的由大肠杆菌中单个基因编码的多聚细胞内溶素结构。粪便噬菌体。因此,它可以为设计针对机会性病原体大肠杆菌的有效细胞内溶素提供有价值的见解。粪便。


























 京公网安备 11010802027423号
京公网安备 11010802027423号