Leukemia ( IF 11.4 ) Pub Date : 2020-03-16 , DOI: 10.1038/s41375-020-0773-5 Pau Montesinos , Gail J. Roboz , Claude-Eric Bulabois , Marion Subklewe , Uwe Platzbecker , Yishai Ofran , Cristina Papayannidis , Agnieszka Wierzbowska , Ho Jin Shin , Vadim Doronin , Stefan Deneberg , Su-Peng Yeh , Mehmet Ali Ozcan , Steven Knapper , Jorge Cortes , Daniel A. Pollyea , Gert Ossenkoppele , Sergio Giralt , Hartmut Döhner , Michael Heuser , Liang Xiu , Indrajeet Singh , Fei Huang , Julie S. Larsen , Andrew H. Wei
|
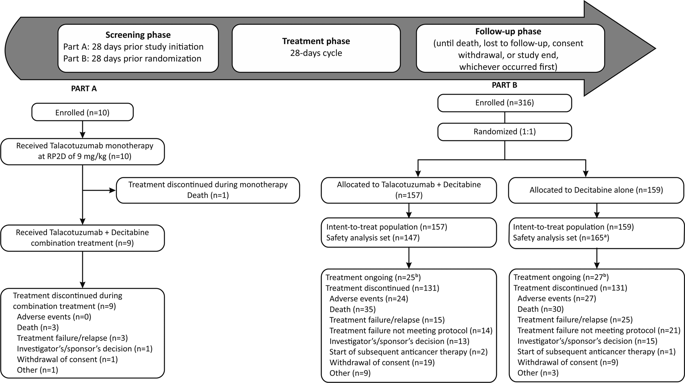
Talacotuzumab, a humanized anti-CD123 monoclonal antibody, was evaluated in combination with decitabine in elderly patients with acute myeloid leukemia (AML) not eligible for intensive chemotherapy. A multicenter, phase 2/3 study was initiated to determine the recommended phase 2 dose (RP2D) of talacotuzumab (Part A) followed by an open-label, randomized comparison of talacotuzumab in combination with decitabine versus decitabine alone to assess achievement of complete response (CR) and overall survival (OS) in Part B. Ten patients were enrolled in Part A and 316 in Part B; the results presented here are based on a database lock on January 25, 2018. Part A confirmed the RP2D of talacotuzumab to be 9 mg/kg. In Part B, CR was achieved in 12/80 (15%) patients receiving combination therapy and in 9/82 (11%) patients receiving decitabine alone (odds ratio: 1.4; 95% confidence interval [CI]: 0.6–3.6; p = 0.44). Median (95% CI) OS was 5.36 (4.27–7.95) months for combination therapy versus 7.26 (6.47–8.64) months for decitabine alone (hazard ratio: 1.04; 95% CI: 0.79–1.37; p = 0.78). Combination therapy showed no improvement in efficacy versus decitabine alone, resulting in the Independent Data Monitoring Committee’s recommendation of early termination of enrollment and discontinuation of talacotuzumab treatment.
中文翻译:

他拉妥珠单抗联合地西他滨或地西他滨单独治疗不适合化疗的急性髓细胞白血病的安全性和有效性:一项多中心,随机,2/3期研究的结果
在不适合强化化疗的老年急性髓细胞性白血病(AML)老年患者中,评估了Talacotuzumab(人源化抗CD123单克隆抗体)与地西他滨联合治疗的情况。开始了一项多中心的2/3期研究,以确定他曲妥珠单抗的推荐的第2期剂量(RP2D)(A部分),然后对他曲妥珠单抗联合地西他滨与地西他滨的开放标签随机比较,以评估完全缓解的情况B部分的(CR)和总生存期(OS)。A部分招募了10名患者,B部分招募了316名患者。此处显示的结果是基于2018年1月25日的数据库锁定。A部分确认了talacotuzumab的RP2D为9 mg / kg。在B部分中p = 0.44)。联合治疗的中位(95%CI)OS为5.36(4.27–7.95)个月,而单独的地西他滨为7.26(6.47–8.64)个月(危险比:1.04; 95%CI:0.79–1.37;p = 0.78)。与单独使用地西他滨相比,联合治疗并未显示出疗效的改善,因此,独立数据监测委员会(Independent Data Monitoring Committee)提出了一项建议,即应尽早终止研究和中止他拉卡珠单抗的治疗。



























 京公网安备 11010802027423号
京公网安备 11010802027423号