Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Efficacy of a tetravalent dengue vaccine in healthy children aged 4-16 years: a randomised, placebo-controlled, phase 3 trial.
The Lancet ( IF 168.9 ) Pub Date : 2020-03-17 , DOI: 10.1016/s0140-6736(20)30414-1 Shibadas Biswal 1 , Charissa Borja-Tabora 2 , Luis Martinez Vargas 3 , Hector Velásquez 4 , Maria Theresa Alera 5 , Victor Sierra 6 , Edith Johana Rodriguez-Arenales 7 , Delia Yu 8 , V Pujitha Wickramasinghe 9 , Edson Duarte Moreira 10 , Asvini D Fernando 11 , Dulanie Gunasekera 12 , Pope Kosalaraksa 13 , Felix Espinoza 14 , Eduardo López-Medina 15 , Lulu Bravo 16 , Suely Tuboi 17 , Yanee Hutagalung 18 , Pedro Garbes 1 , Ian Escudero 18 , Martina Rauscher 19 , Svetlana Bizjajeva 19 , Inge LeFevre 19 , Astrid Borkowski 19 , Xavier Saez-Llorens 20 , Derek Wallace 1 ,
The Lancet ( IF 168.9 ) Pub Date : 2020-03-17 , DOI: 10.1016/s0140-6736(20)30414-1 Shibadas Biswal 1 , Charissa Borja-Tabora 2 , Luis Martinez Vargas 3 , Hector Velásquez 4 , Maria Theresa Alera 5 , Victor Sierra 6 , Edith Johana Rodriguez-Arenales 7 , Delia Yu 8 , V Pujitha Wickramasinghe 9 , Edson Duarte Moreira 10 , Asvini D Fernando 11 , Dulanie Gunasekera 12 , Pope Kosalaraksa 13 , Felix Espinoza 14 , Eduardo López-Medina 15 , Lulu Bravo 16 , Suely Tuboi 17 , Yanee Hutagalung 18 , Pedro Garbes 1 , Ian Escudero 18 , Martina Rauscher 19 , Svetlana Bizjajeva 19 , Inge LeFevre 19 , Astrid Borkowski 19 , Xavier Saez-Llorens 20 , Derek Wallace 1 ,
Affiliation
|
BACKGROUND
A substantial unmet need remains for safe and effective vaccines against dengue virus disease, particularly for individuals who are dengue-naive and those younger than 9 years. We aimed to assess the efficacy, safety, and immunogenicity of a live attenuated tetravalent dengue vaccine (TAK-003) in healthy children aged 4-16 years.
METHODS
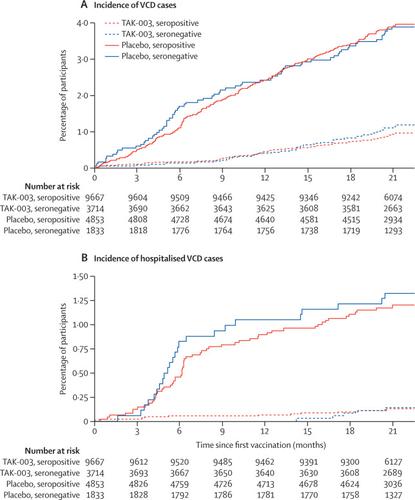
We present data up to 18 months post-vaccination from an ongoing phase 3, randomised, double-blind trial of TAK-003 in endemic regions of Asia and Latin America (26 medical and research centres across Brazil, Colombia, Dominican Republic, Nicaragua, Panama, Philippines, Sri Lanka, and Thailand). Healthy children aged 4-16 years were randomly assigned 2:1 (stratified by age and region) to receive two doses of TAK-003 or two doses of placebo, 3 months apart. Investigators, participants and their parents or guardians, and sponsor representatives advising on trial conduct were masked to trial group assignments. Participants presenting with febrile illness were tested for virologically confirmed dengue (VCD) by serotype-specific RT-PCR. In timeframes beginning 30 days post-second dose, the primary endpoint (overall vaccine efficacy) was assessed in the first 11 months, and the secondary endpoints (efficacy by baseline serostatus, serotype, hospitalised dengue, and severe dengue) in the first 17 months. This study is registered with ClinicalTrials.gov, NCT02747927.
FINDINGS
20 099 participants were randomly assigned and vaccinated between Sept 7, 2016, and Aug 18, 2017; 19 021 (94·6%) were included in the per protocol analysis, and 20 071 (99·9%) in the safety set. The primary endpoint was achieved with an overall vaccine efficacy of 80·2% (95% CI 73·3 to 85·3; 61 cases of VCD in the TAK-003 group vs 149 cases of VCD in the placebo group). In the secondary endpoint assessment timeframe, an overall vaccine efficacy of 73·3% (95% CI 66·5 to 78·8) was observed. Analysis of secondary endpoints showed efficacies of 76·1% (95% CI 68·5 to 81·9) in individuals who were seropositive at baseline, 66·2% (49·1 to 77·5) in individuals who were seronegative at baseline, 90·4% (82·6 to 94·7) against hospitalised dengue, and 85·9% (31·9 to 97·1) against dengue haemorrhagic fever. Efficacy varied by individual serotypes (DENV 1, 69·8% [95% CI 54·8 to 79·9]; DENV 2, 95·1% [89·9 to 97·6]; DENV 3, 48·9% [27·2 to 64·1]; DENV 4, 51·0% [-69·4 to 85·8]). Cumulative rates of serious adverse events were similar in TAK-003 (4·0%) and placebo (4·8%) recipients, and were consistent with expected medical disorders in the study population. Infection was the most frequent reason leading to serious adverse events. 20 participants (<0·1% of the safety set) were withdrawn from the trial due to 21 adverse events by the end of part two; 14 of these participants received TAK-003 and six received placebo.
INTERPRETATION
TAK-003 was well tolerated and efficacious against symptomatic dengue in children regardless of serostatus before immunisation. Vaccine efficacy varied by serotype, warranting continued follow-up to assess longer-term vaccine performance.
FUNDING
Takeda Vaccines.
中文翻译:

四价登革热疫苗在4-16岁健康儿童中的功效:一项随机,安慰剂对照的3期临床试验。
背景技术仍然存在对针对登革热病毒疾病的安全和有效疫苗的大量未满足的需求,特别是针对未登革热且年龄小于9岁的人。我们旨在评估4-16岁健康儿童的减毒活四价登革热疫苗(TAK-003)的功效,安全性和免疫原性。方法我们提供了正在进行的TAK-003在亚洲和拉丁美洲的流行地区(巴西,哥伦比亚,多米尼加共和国,尼加拉瓜的26个医学和研究中心)正在进行的第三阶段,随机双盲试验的疫苗接种后长达18个月的数据,巴拿马,菲律宾,斯里兰卡和泰国)。4-16岁的健康儿童被随机分配为2:1(按年龄和地区分层),分别接受两剂TAK-003或两剂安慰剂,相隔3个月。调查员 参加者,他们的父母或监护人,以及就审判行为提供建议的赞助商代表被掩盖在审判小组的任务中。通过血清型特异性RT-PCR对出现高热疾病的参与者进行病毒学证实的登革热(VCD)测试。在第二次给药后30天开始的时间范围内,在前11个月评估主要终点(总体疫苗效力),并在前17个月评估次要终点(基线血清状况,血清型,登革热和重度登革热的疗效) 。该研究已在ClinicalTrials.gov注册,NCT02747927。调查结果在2016年9月7日至2017年8月18日之间,随机分配了20 099名参与者并进行了疫苗接种;每个方案分析中包括19 021(94·6%),安全集中包括20 071(99·9%)。达到主要终点的总体疫苗效力为80·2%(95%CI 73·3至85·3; TAK-003组为VCD的61例,而安慰剂组为149的VCD)。在次要终点评估时间范围内,观察到总体疫苗效力为73·3%(95%CI 66·5至78·8)。次要终点的分析显示,在基线时呈血清阳性的个体的效率为76·1%(95%CI 68·5至81·9),在血清呈阴性的个体中的效率为66·2%(49·1至77·5)。基线,针对住院登革热的比率为90·4%(82·6至94·7),针对登革出血热的比率为85·9%(31·9至97·1)。效率因个体血清型而异(DENV 1、69·8%[95%CI 54·8至79·9]; DENV 2、95·1%[89·9至97·6]; DENV 3、48·9% [27·2至64·1]; DENV 4、51·0%[-69·4至85·8])。在TAK-003(4·0%)和安慰剂(4·8%)的接受者中,严重不良事件的累积发生率相似,并且与研究人群的预期医学疾病相符。感染是导致严重不良事件的最常见原因。到第二部分结束时,由于21种不良事件,有20名参与者(安全设置的<0·1%)退出了试验;这些参与者中有14名接受了TAK-003的接受,而六名接受了安慰剂。解释TAK-003对儿童的症状性登革热具有良好的耐受性和免疫力,而与免疫前的血清状况无关。疫苗的功效因血清型而异,需要继续随访以评估长期疫苗的性能。资助武田疫苗。感染是导致严重不良事件的最常见原因。到第二部分结束时,由于21种不良事件,有20名参与者(安全设置的<0·1%)退出了试验;这些参与者中有14名接受了TAK-003的接受,而六名接受了安慰剂。解释TAK-003对儿童的症状性登革热具有良好的耐受性和免疫力,无论免疫前血清状况如何。疫苗的功效因血清型而异,需要继续随访以评估长期疫苗的性能。资助武田疫苗。感染是导致严重不良事件的最常见原因。到第二部分结束时,由于21种不良事件,有20名参与者(<0.1%的安全性)退出了试验;这些参与者中有14名接受了TAK-003的接受,而六名接受了安慰剂。解释TAK-003对儿童的症状性登革热耐受性好,对免疫接种前的血清状态无关。疫苗的功效因血清型而异,需要继续随访以评估长期疫苗的性能。资助武田疫苗。解释TAK-003对儿童的症状性登革热耐受性好,对免疫接种前的血清状态无关。疫苗的功效因血清型而异,需要继续随访以评估长期疫苗的性能。资助武田疫苗。解释TAK-003对儿童的症状性登革热具有良好的耐受性和免疫力,而与免疫前的血清状况无关。疫苗的功效因血清型而异,需要继续随访以评估长期疫苗的性能。资助武田疫苗。
更新日期:2020-04-22
中文翻译:

四价登革热疫苗在4-16岁健康儿童中的功效:一项随机,安慰剂对照的3期临床试验。
背景技术仍然存在对针对登革热病毒疾病的安全和有效疫苗的大量未满足的需求,特别是针对未登革热且年龄小于9岁的人。我们旨在评估4-16岁健康儿童的减毒活四价登革热疫苗(TAK-003)的功效,安全性和免疫原性。方法我们提供了正在进行的TAK-003在亚洲和拉丁美洲的流行地区(巴西,哥伦比亚,多米尼加共和国,尼加拉瓜的26个医学和研究中心)正在进行的第三阶段,随机双盲试验的疫苗接种后长达18个月的数据,巴拿马,菲律宾,斯里兰卡和泰国)。4-16岁的健康儿童被随机分配为2:1(按年龄和地区分层),分别接受两剂TAK-003或两剂安慰剂,相隔3个月。调查员 参加者,他们的父母或监护人,以及就审判行为提供建议的赞助商代表被掩盖在审判小组的任务中。通过血清型特异性RT-PCR对出现高热疾病的参与者进行病毒学证实的登革热(VCD)测试。在第二次给药后30天开始的时间范围内,在前11个月评估主要终点(总体疫苗效力),并在前17个月评估次要终点(基线血清状况,血清型,登革热和重度登革热的疗效) 。该研究已在ClinicalTrials.gov注册,NCT02747927。调查结果在2016年9月7日至2017年8月18日之间,随机分配了20 099名参与者并进行了疫苗接种;每个方案分析中包括19 021(94·6%),安全集中包括20 071(99·9%)。达到主要终点的总体疫苗效力为80·2%(95%CI 73·3至85·3; TAK-003组为VCD的61例,而安慰剂组为149的VCD)。在次要终点评估时间范围内,观察到总体疫苗效力为73·3%(95%CI 66·5至78·8)。次要终点的分析显示,在基线时呈血清阳性的个体的效率为76·1%(95%CI 68·5至81·9),在血清呈阴性的个体中的效率为66·2%(49·1至77·5)。基线,针对住院登革热的比率为90·4%(82·6至94·7),针对登革出血热的比率为85·9%(31·9至97·1)。效率因个体血清型而异(DENV 1、69·8%[95%CI 54·8至79·9]; DENV 2、95·1%[89·9至97·6]; DENV 3、48·9% [27·2至64·1]; DENV 4、51·0%[-69·4至85·8])。在TAK-003(4·0%)和安慰剂(4·8%)的接受者中,严重不良事件的累积发生率相似,并且与研究人群的预期医学疾病相符。感染是导致严重不良事件的最常见原因。到第二部分结束时,由于21种不良事件,有20名参与者(安全设置的<0·1%)退出了试验;这些参与者中有14名接受了TAK-003的接受,而六名接受了安慰剂。解释TAK-003对儿童的症状性登革热具有良好的耐受性和免疫力,而与免疫前的血清状况无关。疫苗的功效因血清型而异,需要继续随访以评估长期疫苗的性能。资助武田疫苗。感染是导致严重不良事件的最常见原因。到第二部分结束时,由于21种不良事件,有20名参与者(安全设置的<0·1%)退出了试验;这些参与者中有14名接受了TAK-003的接受,而六名接受了安慰剂。解释TAK-003对儿童的症状性登革热具有良好的耐受性和免疫力,无论免疫前血清状况如何。疫苗的功效因血清型而异,需要继续随访以评估长期疫苗的性能。资助武田疫苗。感染是导致严重不良事件的最常见原因。到第二部分结束时,由于21种不良事件,有20名参与者(<0.1%的安全性)退出了试验;这些参与者中有14名接受了TAK-003的接受,而六名接受了安慰剂。解释TAK-003对儿童的症状性登革热耐受性好,对免疫接种前的血清状态无关。疫苗的功效因血清型而异,需要继续随访以评估长期疫苗的性能。资助武田疫苗。解释TAK-003对儿童的症状性登革热耐受性好,对免疫接种前的血清状态无关。疫苗的功效因血清型而异,需要继续随访以评估长期疫苗的性能。资助武田疫苗。解释TAK-003对儿童的症状性登革热具有良好的耐受性和免疫力,而与免疫前的血清状况无关。疫苗的功效因血清型而异,需要继续随访以评估长期疫苗的性能。资助武田疫苗。

























 京公网安备 11010802027423号
京公网安备 11010802027423号