当前位置:
X-MOL 学术
›
Eur. J. Pharm. Biopharm.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Relationship of PEG-induced precipitation with protein-protein interactions and aggregation rates of high concentration mAb formulations at 5 °C.
European Journal of Pharmaceutics and Biopharmaceutics ( IF 4.9 ) Pub Date : 2020-03-18 , DOI: 10.1016/j.ejpb.2020.03.011 Ruben Wälchli 1 , Francesca Fanizzi 1 , Jan Massant 2 , Paolo Arosio 1
European Journal of Pharmaceutics and Biopharmaceutics ( IF 4.9 ) Pub Date : 2020-03-18 , DOI: 10.1016/j.ejpb.2020.03.011 Ruben Wälchli 1 , Francesca Fanizzi 1 , Jan Massant 2 , Paolo Arosio 1
Affiliation
|
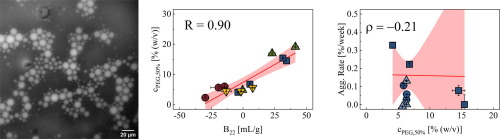
Native protein-protein interactions can play an important role in determining the tendency of monoclonal antibodies (mAbs) to aggregate under storage conditions. In this context, phase separation of mAb solutions induced by the addition of neutral polymers such as poly(ethylene glycol) (PEG) represents a simple method to assess the tendency of proteins to self-associate in the native state. Here, we investigated their relationships between PEG-induced phase separation, protein-protein interactions and long-term aggregation rate of several formulations of four mAbs at 100 mg/mL and 5 °C over 12 weeks of storage. We observed that the location of the phase boundary correlated well with the osmotic second virial coefficient B22 determined in absence of the polymer, indicating that for our solutions PEG primarily leads to depletion forces between protein molecules, which are additive to protein-protein interactions. However, limited correlation between aggregation rate at 5 °C and phase behavior was observed across different mAbs, pH values and ionic strengths, indicating that colloidal stability is not the only determinant of aggregation even at such low temperature and high protein concentration. Our results contribute to the growing realization that aggregation propensity in the context of antibody developability is a complex feature, which depends on a variety of biophysical properties rather than one single parameter.
中文翻译:

PEG诱导的沉淀与高浓度mAb制剂在5°C下的蛋白相互作用和聚集速率之间的关系。
天然蛋白质-蛋白质相互作用可以在确定单克隆抗体(mAb)在储存条件下聚集的趋势中发挥重要作用。在这种情况下,通过添加中性聚合物(例如聚(乙二醇)(PEG))诱导的mAb溶液的相分离代表了一种简单的方法,可以评估蛋白质在天然状态下自缔合的趋势。在这里,我们研究了在储存12周内100 mg / mL和5°C下四种mAb的几种制剂在PEG诱导的相分离,蛋白-蛋白相互作用和长期聚集率之间的关系。我们观察到,相边界的位置与不存在聚合物时确定的渗透第二维里系数B22密切相关,表明对于我们的解决方案,PEG主要导致蛋白质分子之间的耗竭力,这是蛋白质与蛋白质相互作用的加成。但是,在不同的mAb,pH值和离子强度下,观察到5°C的聚集速率与相行为之间的相关性有限,这表明即使在如此低的温度和较高的蛋白质浓度下,胶体稳定性也不是聚集的唯一决定因素。我们的结果有助于人们逐渐认识到,在抗体可开发性的背景下,聚集倾向是一个复杂的特征,它取决于多种生物物理特性,而不是一个参数。pH值和离子强度,表明即使在如此低的温度和较高的蛋白质浓度下,胶体稳定性也不是聚集的唯一决定因素。我们的结果有助于人们逐渐认识到,在抗体可开发性的背景下,聚集倾向是一个复杂的特征,它取决于多种生物物理特性,而不是单个参数。pH值和离子强度,表明即使在如此低的温度和较高的蛋白质浓度下,胶体稳定性也不是唯一的聚集决定因素。我们的结果有助于人们逐渐认识到,在抗体可开发性的背景下,聚集倾向是一个复杂的特征,它取决于多种生物物理特性,而不是一个参数。
更新日期:2020-03-19
中文翻译:

PEG诱导的沉淀与高浓度mAb制剂在5°C下的蛋白相互作用和聚集速率之间的关系。
天然蛋白质-蛋白质相互作用可以在确定单克隆抗体(mAb)在储存条件下聚集的趋势中发挥重要作用。在这种情况下,通过添加中性聚合物(例如聚(乙二醇)(PEG))诱导的mAb溶液的相分离代表了一种简单的方法,可以评估蛋白质在天然状态下自缔合的趋势。在这里,我们研究了在储存12周内100 mg / mL和5°C下四种mAb的几种制剂在PEG诱导的相分离,蛋白-蛋白相互作用和长期聚集率之间的关系。我们观察到,相边界的位置与不存在聚合物时确定的渗透第二维里系数B22密切相关,表明对于我们的解决方案,PEG主要导致蛋白质分子之间的耗竭力,这是蛋白质与蛋白质相互作用的加成。但是,在不同的mAb,pH值和离子强度下,观察到5°C的聚集速率与相行为之间的相关性有限,这表明即使在如此低的温度和较高的蛋白质浓度下,胶体稳定性也不是聚集的唯一决定因素。我们的结果有助于人们逐渐认识到,在抗体可开发性的背景下,聚集倾向是一个复杂的特征,它取决于多种生物物理特性,而不是一个参数。pH值和离子强度,表明即使在如此低的温度和较高的蛋白质浓度下,胶体稳定性也不是聚集的唯一决定因素。我们的结果有助于人们逐渐认识到,在抗体可开发性的背景下,聚集倾向是一个复杂的特征,它取决于多种生物物理特性,而不是单个参数。pH值和离子强度,表明即使在如此低的温度和较高的蛋白质浓度下,胶体稳定性也不是唯一的聚集决定因素。我们的结果有助于人们逐渐认识到,在抗体可开发性的背景下,聚集倾向是一个复杂的特征,它取决于多种生物物理特性,而不是一个参数。


























 京公网安备 11010802027423号
京公网安备 11010802027423号