当前位置:
X-MOL 学术
›
Acta Biomater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Tumor necrosis factor primes and metal particles activate the NLRP3 inflammasome in human primary macrophages.
Acta Biomaterialia ( IF 9.7 ) Pub Date : 2020-03-17 , DOI: 10.1016/j.actbio.2020.03.017 Eemeli Jämsen 1 , Jukka Pajarinen 1 , Vesa-Petteri Kouri 1 , Antti Rahikkala 2 , Stuart B Goodman 3 , Mikko Manninen 4 , Dan C Nordström 5 , Kari K Eklund 6 , Katariina Nurmi 1
Acta Biomaterialia ( IF 9.7 ) Pub Date : 2020-03-17 , DOI: 10.1016/j.actbio.2020.03.017 Eemeli Jämsen 1 , Jukka Pajarinen 1 , Vesa-Petteri Kouri 1 , Antti Rahikkala 2 , Stuart B Goodman 3 , Mikko Manninen 4 , Dan C Nordström 5 , Kari K Eklund 6 , Katariina Nurmi 1
Affiliation
|
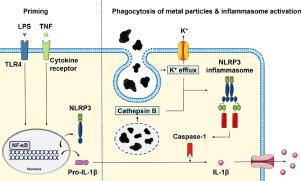
Aseptic loosening of total joint replacements is driven by a macrophage-mediated inflammatory reaction to implant-derived wear particles. Phagocytosis of implant debris has been suggested to activate the NLRP3 inflammasome leading to secretion of interleukin (IL)-1β. However, factors and molecular mechanisms driving the particle-induced inflammasome activation are yet to be fully elucidated. In this study, we investigated the inflammasome response of human primary macrophages to titanium, chromium, and molybdenum particles in vitro. We observed that particles alone were not sufficient to induce IL-1β secretion, but an additional priming signal-such as bacterial lipopolysaccharide (LPS)-was required to license the inflammasome activation. By using specific inhibitors against the inflammasome signaling pathway, we demonstrate that the particle-induced IL-1β secretion depended upon activation of the NLRP3 inflammasome. We further hypothesized that tumor necrosis factor (TNF) could substitute for LPS as a priming signal, and found that particle stimulation together with preceding TNF treatment resulted in inflammasome-dependent IL-1β production as well. Our results show that the NLRP3 inflammasome mediates wear particle responses in human primary macrophages, and its activation does not necessarily require the presence of bacterial components, but can be induced under aseptic conditions by TNF priming. STATEMENT OF SIGNIFICANCE: This study was conducted to elucidate the molecular mechanisms of metal particle-induced IL-1β secretion in human primary macrophages. Production of this pro-inflammatory mediator from wear particle-activated macrophages has been associated with increased bone loss around total joint replacements-a condition eventually requiring revision surgery. Our results confirm that together with a co-stimulatory priming signal, particles of common implant metals elicit macrophage-mediated IL-1β secretion through activation of the NLRP3 inflammasome pathway. We also present a concept of TNF priming in this context, demonstrating that the particle-related IL-1β secretion can take place in a truly sterile environment. Thus, inhibition of inflammasome signaling appears a means to prevent wear particle-induced inflammation and development of peri‑prosthetic osteolysis.
中文翻译:

肿瘤坏死因子引发剂和金属颗粒激活人原代巨噬细胞中的 NLRP3 炎性体。
全关节置换的无菌性松动是由巨噬细胞介导的对植入物磨损颗粒的炎症反应驱动的。已经建议吞噬植入物碎片以激活 NLRP3 炎性体,导致白细胞介素 (IL)-1β 的分泌。然而,驱动粒子诱导的炎性体激活的因素和分子机制尚未完全阐明。在这项研究中,我们在体外研究了人类原代巨噬细胞对钛、铬和钼颗粒的炎症反应。我们观察到单独的颗粒不足以诱导 IL-1β 分泌,但需要额外的启动信号——如细菌脂多糖 (LPS)——来激活炎性体。通过使用针对炎症小体信号通路的特异性抑制剂,我们证明了颗粒诱导的 IL-1β 分泌依赖于 NLRP3 炎性体的激活。我们进一步假设肿瘤坏死因子 (TNF) 可以替代 LPS 作为启动信号,并发现粒子刺激与之前的 TNF 治疗一起导致炎症小体依赖性 IL-1β 的产生。我们的研究结果表明,NLRP3 炎性体介导人类原代巨噬细胞的磨损颗粒反应,其激活不一定需要细菌成分的存在,但可以在无菌条件下通过 TNF 引发来诱导。意义声明:本研究旨在阐明金属颗粒诱导人原代巨噬细胞分泌 IL-1β 的分子机制。这种由磨损颗粒激活的巨噬细胞产生的促炎介质与全关节置换周围的骨质流失增加有关——这种情况最终需要进行翻修手术。我们的结果证实,与共刺激启动信号一起,常见植入金属颗粒通过激活 NLRP3 炎性体途径引发巨噬细胞介导的 IL-1β 分泌。在这种情况下,我们还提出了 TNF 引发的概念,证明与颗粒相关的 IL-1β 分泌可以在真正无菌的环境中发生。因此,抑制炎性体信号传导似乎是防止磨损颗粒引起的炎症和假体周围骨溶解发展的一种手段。我们的结果证实,与共刺激启动信号一起,常见植入金属颗粒通过激活 NLRP3 炎性体途径引发巨噬细胞介导的 IL-1β 分泌。在这种情况下,我们还提出了 TNF 引发的概念,证明与颗粒相关的 IL-1β 分泌可以在真正无菌的环境中发生。因此,抑制炎性体信号传导似乎是防止磨损颗粒引起的炎症和假体周围骨溶解发展的一种手段。我们的结果证实,与共刺激启动信号一起,常见植入金属颗粒通过激活 NLRP3 炎性体途径引发巨噬细胞介导的 IL-1β 分泌。在这种情况下,我们还提出了 TNF 引发的概念,证明与颗粒相关的 IL-1β 分泌可以在真正无菌的环境中发生。因此,抑制炎性体信号传导似乎是防止磨损颗粒引起的炎症和假体周围骨溶解发展的一种手段。证明粒子相关的 IL-1β 分泌可以在真正无菌的环境中发生。因此,抑制炎性体信号传导似乎是防止磨损颗粒引起的炎症和假体周围骨溶解发展的一种手段。证明粒子相关的 IL-1β 分泌可以在真正无菌的环境中发生。因此,抑制炎性体信号传导似乎是防止磨损颗粒引起的炎症和假体周围骨溶解发展的一种手段。
更新日期:2020-03-17
中文翻译:

肿瘤坏死因子引发剂和金属颗粒激活人原代巨噬细胞中的 NLRP3 炎性体。
全关节置换的无菌性松动是由巨噬细胞介导的对植入物磨损颗粒的炎症反应驱动的。已经建议吞噬植入物碎片以激活 NLRP3 炎性体,导致白细胞介素 (IL)-1β 的分泌。然而,驱动粒子诱导的炎性体激活的因素和分子机制尚未完全阐明。在这项研究中,我们在体外研究了人类原代巨噬细胞对钛、铬和钼颗粒的炎症反应。我们观察到单独的颗粒不足以诱导 IL-1β 分泌,但需要额外的启动信号——如细菌脂多糖 (LPS)——来激活炎性体。通过使用针对炎症小体信号通路的特异性抑制剂,我们证明了颗粒诱导的 IL-1β 分泌依赖于 NLRP3 炎性体的激活。我们进一步假设肿瘤坏死因子 (TNF) 可以替代 LPS 作为启动信号,并发现粒子刺激与之前的 TNF 治疗一起导致炎症小体依赖性 IL-1β 的产生。我们的研究结果表明,NLRP3 炎性体介导人类原代巨噬细胞的磨损颗粒反应,其激活不一定需要细菌成分的存在,但可以在无菌条件下通过 TNF 引发来诱导。意义声明:本研究旨在阐明金属颗粒诱导人原代巨噬细胞分泌 IL-1β 的分子机制。这种由磨损颗粒激活的巨噬细胞产生的促炎介质与全关节置换周围的骨质流失增加有关——这种情况最终需要进行翻修手术。我们的结果证实,与共刺激启动信号一起,常见植入金属颗粒通过激活 NLRP3 炎性体途径引发巨噬细胞介导的 IL-1β 分泌。在这种情况下,我们还提出了 TNF 引发的概念,证明与颗粒相关的 IL-1β 分泌可以在真正无菌的环境中发生。因此,抑制炎性体信号传导似乎是防止磨损颗粒引起的炎症和假体周围骨溶解发展的一种手段。我们的结果证实,与共刺激启动信号一起,常见植入金属颗粒通过激活 NLRP3 炎性体途径引发巨噬细胞介导的 IL-1β 分泌。在这种情况下,我们还提出了 TNF 引发的概念,证明与颗粒相关的 IL-1β 分泌可以在真正无菌的环境中发生。因此,抑制炎性体信号传导似乎是防止磨损颗粒引起的炎症和假体周围骨溶解发展的一种手段。我们的结果证实,与共刺激启动信号一起,常见植入金属颗粒通过激活 NLRP3 炎性体途径引发巨噬细胞介导的 IL-1β 分泌。在这种情况下,我们还提出了 TNF 引发的概念,证明与颗粒相关的 IL-1β 分泌可以在真正无菌的环境中发生。因此,抑制炎性体信号传导似乎是防止磨损颗粒引起的炎症和假体周围骨溶解发展的一种手段。证明粒子相关的 IL-1β 分泌可以在真正无菌的环境中发生。因此,抑制炎性体信号传导似乎是防止磨损颗粒引起的炎症和假体周围骨溶解发展的一种手段。证明粒子相关的 IL-1β 分泌可以在真正无菌的环境中发生。因此,抑制炎性体信号传导似乎是防止磨损颗粒引起的炎症和假体周围骨溶解发展的一种手段。


























 京公网安备 11010802027423号
京公网安备 11010802027423号