Tetrahedron ( IF 2.1 ) Pub Date : 2020-03-17 , DOI: 10.1016/j.tet.2020.131143 Yanyan Xu , Chenze Qi , Chen Wang
|
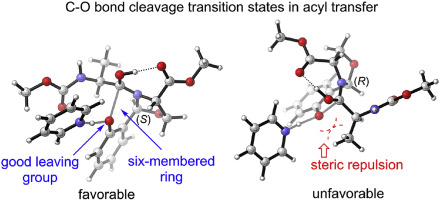
The mechanism of serine/threonine ligation was investigated by density functional theory. The reaction sequentially proceeds through imine capture, 5-endo-trig cyclization and [1,5] O-to-N acyl transfer steps, all of which occur with the assistance of pyridinium. The imine capture and 5-endo-trig cyclization steps are reversible whereas the [1,5] O-to-N acyl transfer step is irreversible. The [1,5] O-to-N acyl transfer step proceeds through a stepwise addition-elimination mechanism with the C–O bond cleavage as the rate-determining step of the overall reaction. The diastereoselectivity for the exclusive formation of the S-configured N,O-benzylidene acetal-linked peptide originates from the disfavored steric repulsion between the leaving phenolic oxygen and the side chain of the C-terminal peptide in the R-configured C–O bond cleavage transition state in the acyl transfer step. A suitable ring size as well as a good leaving group in acyl transfer are responsible for the high efficiency of the serine/threonine ligation reaction.
中文翻译:

非对映选择性的机理,起源和影响丝氨酸/苏氨酸结扎反应效率的因素:计算研究
通过密度泛函理论研究了丝氨酸/苏氨酸连接的机理。反应依次经由亚胺捕获,前进5 -内trig的环化和[1,5] ø -到- ñ酰基转移步骤,所有这些都与吡啶鎓的协助下进行。亚胺捕获和5 -内trig的环化步骤是可逆的,而[1,5] ø -到- ñ酰基转移步骤是不可逆的。在[1,5] ø -到- ñ酰基转移步骤通过与C-O键裂解为总反应的速率决定步骤逐步加成-消除机构进行。排他性形成非对映选择性S构型的N,O-亚苄基缩醛连接的肽源于酰基转移步骤中R构型的C–O键裂解过渡态中离开的酚氧与C端肽的侧链之间的空间排斥性差。合适的环大小以及酰基转移中的良好离去基团是丝氨酸/苏氨酸连接反应高效的原因。


























 京公网安备 11010802027423号
京公网安备 11010802027423号