当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Molecular Docking Studies of Royleanone Diterpenoids from Plectranthus spp. as P-Glycoprotein Inhibitors.
ACS Medicinal Chemistry Letters ( IF 4.2 ) Pub Date : 2020-03-12 , DOI: 10.1021/acsmedchemlett.9b00642 Vera M S Isca 1, 2 , Ricardo J Ferreira 2, 3 , Catarina Garcia 1, 4 , Carlos M Monteiro 2 , Jelena Dinic 5 , Suvi Holmstedt 6 , Vânia André 7 , Milica Pesic 5 , Daniel J V A Dos Santos 2, 8 , Nuno R Candeias 6, 9 , Carlos A M Afonso 2 , Patrícia Rijo 1, 2
ACS Medicinal Chemistry Letters ( IF 4.2 ) Pub Date : 2020-03-12 , DOI: 10.1021/acsmedchemlett.9b00642 Vera M S Isca 1, 2 , Ricardo J Ferreira 2, 3 , Catarina Garcia 1, 4 , Carlos M Monteiro 2 , Jelena Dinic 5 , Suvi Holmstedt 6 , Vânia André 7 , Milica Pesic 5 , Daniel J V A Dos Santos 2, 8 , Nuno R Candeias 6, 9 , Carlos A M Afonso 2 , Patrícia Rijo 1, 2
Affiliation
|
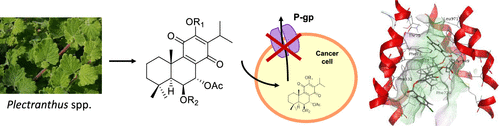
The development of multidrug resistance (MDR) is a major cause of failure in cancer chemotherapy. Several abietane diterpenes with antitumoral activities have been isolated from Plectranthus spp. such as 6,7-dehydroroyleanone (DHR, 1) and 7α-acetoxy-6β-hydroxyroyleanone (AHR, 2). Several royleanone derivatives were prepared through hemisynthesis from natural compounds 1 and 2 to achieve a small library of products with enhanced anti-P-glycoprotein activity. Nonetheless, some derivatives tend to be unstable. Therefore, to reason such lack of stability, the electron density based local reactivity descriptors condensed Fukui functions and dual descriptor were calculated for several derivatives of DHR. Additionally, molecular docking and molecular dynamics studies were performed on several other derivatives to clarify the molecular mechanisms by which they may exert their inhibitory effect in P-gp activity. The analysis on local reactivity descriptors was important to understand possible degradation pathways and to guide further synthetic approaches toward new royleanone derivatives. A molecular docking study suggested that the presence of aromatic moieties increases the binding affinity of royleanone derivatives toward P-gp. It further suggests that one royleanone benzoylated derivative may act as a noncompetitive efflux modulator when bound to the M-site. The future generation of novel royleanone derivatives will involve (i) a selective modification of position C-12 with chemical moieties smaller than unsubstituted benzoyl rings and (ii) the modification of the substitution pattern of the benzoyloxy moiety at position C-6.
中文翻译:

Plectranthus spp。的鼠李酮二萜类化合物的分子对接研究。作为P-糖蛋白抑制剂。
多药耐药性(MDR)的发展是癌症化疗失败的主要原因。已经从Plectranthus spp中分离出几种具有抗肿瘤活性的松果糖二萜。如6,7-脱氢鲁拉酮(DHR,1)和7α-乙酰氧基-6β-羟基鲁拉酮(AHR,2)。通过半合成从天然化合物1和2制备了几种萝莉酮衍生物,以获得具有增强的抗P-糖蛋白活性的小型产品文库。但是,某些导数往往不稳定。因此,由于这种缺乏稳定性的原因,针对DHR的几种导数,计算了基于电子密度的局部反应性描述子,其中包含了简化的Fukui函数和双重描述子。另外,对其他几种衍生物进行了分子对接和分子动力学研究,以阐明其可能在P-gp活性中发挥抑制作用的分子机制。对局部反应性描述子的分析对于理解可能的降解途径和指导进一步的合成方法制备新的丙酮酸酮衍生物具有重要意义。一项分子对接研究表明,芳香族基团的存在增加了胭脂酮衍生物对P-gp的结合亲和力。这进一步表明,当与M-位点结合时,一种丙酮酸酮基苯甲酰化衍生物可以充当非竞争性外排调节剂。
更新日期:2020-03-12
中文翻译:

Plectranthus spp。的鼠李酮二萜类化合物的分子对接研究。作为P-糖蛋白抑制剂。
多药耐药性(MDR)的发展是癌症化疗失败的主要原因。已经从Plectranthus spp中分离出几种具有抗肿瘤活性的松果糖二萜。如6,7-脱氢鲁拉酮(DHR,1)和7α-乙酰氧基-6β-羟基鲁拉酮(AHR,2)。通过半合成从天然化合物1和2制备了几种萝莉酮衍生物,以获得具有增强的抗P-糖蛋白活性的小型产品文库。但是,某些导数往往不稳定。因此,由于这种缺乏稳定性的原因,针对DHR的几种导数,计算了基于电子密度的局部反应性描述子,其中包含了简化的Fukui函数和双重描述子。另外,对其他几种衍生物进行了分子对接和分子动力学研究,以阐明其可能在P-gp活性中发挥抑制作用的分子机制。对局部反应性描述子的分析对于理解可能的降解途径和指导进一步的合成方法制备新的丙酮酸酮衍生物具有重要意义。一项分子对接研究表明,芳香族基团的存在增加了胭脂酮衍生物对P-gp的结合亲和力。这进一步表明,当与M-位点结合时,一种丙酮酸酮基苯甲酰化衍生物可以充当非竞争性外排调节剂。


























 京公网安备 11010802027423号
京公网安备 11010802027423号