当前位置:
X-MOL 学术
›
ACS Infect. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Searching for Antipneumococcal Targets: Choline-Binding Modules as Phagocytosis Enhancers.
ACS Infectious Diseases ( IF 5.3 ) Pub Date : 2020-03-17 , DOI: 10.1021/acsinfecdis.9b00344 Emma Roig-Molina 1 , Manuel Sánchez-Angulo 2 , Jana Seele 3, 4 , Francisco García-Asencio 1 , Roland Nau 3, 4 , Jesús M Sanz 1, 5, 6 , Beatriz Maestro 1, 5
ACS Infectious Diseases ( IF 5.3 ) Pub Date : 2020-03-17 , DOI: 10.1021/acsinfecdis.9b00344 Emma Roig-Molina 1 , Manuel Sánchez-Angulo 2 , Jana Seele 3, 4 , Francisco García-Asencio 1 , Roland Nau 3, 4 , Jesús M Sanz 1, 5, 6 , Beatriz Maestro 1, 5
Affiliation
|
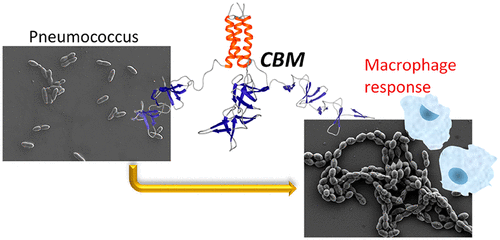
Choline-binding proteins (CBPs) from Streptococcus pneumoniae comprise a family of modular polypeptides involved in essential events of this pathogen. They recognize the choline residues present in the teichoic and lipoteichoic acids of the cell wall using the so-called choline-binding modules (CBMs). The importance of CBPs in pneumococcal physiology points to them as novel targets to combat antimicrobial resistances shown by this organism. In this work we have tested the ability of exogenously added CBMs to act as CBP inhibitors by competing with the latter for the binding to the choline molecules in the bacterial surface. First, we carried out a thorough physicochemical characterization of three native CBMs, namely C-LytA, C-Cpl1, and C-CbpD, and assessed their affinity for choline and macromolecular, pneumococcal cell-wall mimics. The interaction with these substrates was evaluated by molecular modeling, analytical ultracentrifugation, surface plasmon resonance, and fluorescence and circular dichroism spectroscopies. Van't Hoff thermal analyses unveiled the existence of one noncanonical choline binding site in each of the C-Cpl1 and C-CbpD proteins, leading in total to 5 ligand-binding sites per dimer and 4 sites per monomer, respectively. Remarkably, the binding affinities of the CBMs do not directly correlate with their native oligomeric state or with the number of choline-binding sites, suggesting that choline recognition by these modules is a complex phenomenon. On the other hand, the exogenous addition of CBMs to pneumococcal planktonic cultures caused extensive cell-chaining probably as a consequence of the inhibition of CBP attachment to the cell wall. This was accompanied by bacterial aggregation and sedimentation, causing an enhancement of bacterial phagocytosis by peritoneal macrophages. In addition, the rational design of an oligomeric variant of a native CBM led to a substantial increase in its antibacterial activity by multivalency effects. These results suggest that CBMs might constitute promising nonlytic antimicrobial candidates based on the natural induction of the host defense system.
中文翻译:

搜索抗肺炎球菌靶标:胆碱结合模块作为吞噬作用增强剂。
来自肺炎链球菌的胆碱结合蛋白(CBP)包含涉及该病原体必需事件的模块化多肽家族。他们利用所谓的胆碱结合模块(CBM)识别细胞壁的回潮酸和脂蛋白回酸中存在的胆碱残基。CBPs在肺炎球菌生理中的重要性表明,它们是对抗这种生物体显示的抗药性的新靶标。在这项工作中,我们已经测试了外源添加的CBM与CBP抑制剂竞争与细菌表面中胆碱分子的结合的能力。首先,我们对三种天然CBM,即C-LytA,C-Cpl1和C-CbpD进行了彻底的理化表征,并评估了它们对胆碱和大分子,肺炎球菌细胞壁模拟物的亲和力。通过分子建模,分析超速离心,表面等离振子共振以及荧光和圆二色性光谱学评估了与这些底物的相互作用。Van't Hoff热分析揭示了每个C-Cpl1和C-CbpD蛋白中都存在一个非规范的胆碱结合位点,导致每个二聚体总共5个配体结合位点和每个单体4个位点。值得注意的是,CBM的结合亲和力与其天然的低聚状态或胆碱结合位点的数量不直接相关,这表明这些模块对胆碱的识别是一个复杂的现象。另一方面,在肺炎球菌浮游培养物中外源添加CBMs可能导致了广泛的细胞连锁,这可能是由于CBP附着在细胞壁上所致。这伴随着细菌的聚集和沉淀,导致腹膜巨噬细胞增强了细菌的吞噬作用。另外,天然CBM的寡聚变体的合理设计通过多价效应导致其抗菌活性大大增加。这些结果表明,基于宿主防御系统的自然诱导,煤层气可能构成有前途的非溶解性抗菌药物候选物。
更新日期:2020-03-05
中文翻译:

搜索抗肺炎球菌靶标:胆碱结合模块作为吞噬作用增强剂。
来自肺炎链球菌的胆碱结合蛋白(CBP)包含涉及该病原体必需事件的模块化多肽家族。他们利用所谓的胆碱结合模块(CBM)识别细胞壁的回潮酸和脂蛋白回酸中存在的胆碱残基。CBPs在肺炎球菌生理中的重要性表明,它们是对抗这种生物体显示的抗药性的新靶标。在这项工作中,我们已经测试了外源添加的CBM与CBP抑制剂竞争与细菌表面中胆碱分子的结合的能力。首先,我们对三种天然CBM,即C-LytA,C-Cpl1和C-CbpD进行了彻底的理化表征,并评估了它们对胆碱和大分子,肺炎球菌细胞壁模拟物的亲和力。通过分子建模,分析超速离心,表面等离振子共振以及荧光和圆二色性光谱学评估了与这些底物的相互作用。Van't Hoff热分析揭示了每个C-Cpl1和C-CbpD蛋白中都存在一个非规范的胆碱结合位点,导致每个二聚体总共5个配体结合位点和每个单体4个位点。值得注意的是,CBM的结合亲和力与其天然的低聚状态或胆碱结合位点的数量不直接相关,这表明这些模块对胆碱的识别是一个复杂的现象。另一方面,在肺炎球菌浮游培养物中外源添加CBMs可能导致了广泛的细胞连锁,这可能是由于CBP附着在细胞壁上所致。这伴随着细菌的聚集和沉淀,导致腹膜巨噬细胞增强了细菌的吞噬作用。另外,天然CBM的寡聚变体的合理设计通过多价效应导致其抗菌活性大大增加。这些结果表明,基于宿主防御系统的自然诱导,煤层气可能构成有前途的非溶解性抗菌药物候选物。


























 京公网安备 11010802027423号
京公网安备 11010802027423号