当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The Influence of the Ligand in the Iridium Mediated Electrocatalyic Water Oxidation
ACS Catalysis ( IF 12.9 ) Pub Date : 2020-03-25 , DOI: 10.1021/acscatal.0c00531 Bas van Dijk 1 , Gabriel Menendez Rodriguez 2 , Longfei Wu 3 , Jan P. Hofmann 3 , Alceo Macchioni 2 , Dennis G. H. Hetterscheid 1
ACS Catalysis ( IF 12.9 ) Pub Date : 2020-03-25 , DOI: 10.1021/acscatal.0c00531 Bas van Dijk 1 , Gabriel Menendez Rodriguez 2 , Longfei Wu 3 , Jan P. Hofmann 3 , Alceo Macchioni 2 , Dennis G. H. Hetterscheid 1
Affiliation
|
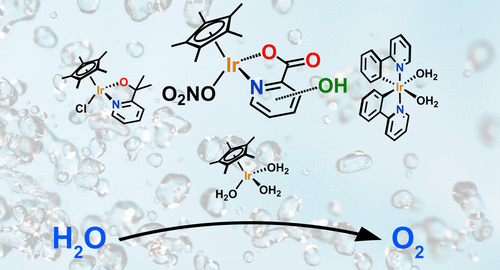
Electrochemical water oxidation is the bottleneck of electrolyzers as even the best catalysts, iridium and ruthenium oxides, have to operate at significant overpotentials. Previously, the position of a hydroxyl on a series of hydroxylpicolinate ligands was found to significantly influence the activity of molecular iridium catalysts in sacrificial oxidant driven water oxidation. In this study, these catalysts were tested under electrochemical conditions and benchmarked to several other known molecular iridium catalysts under the exact same conditions. This allowed us to compare these catalysts directly and observe whether structure–activity relationships would prevail under electrochemical conditions. Using both electrochemical quartz crystal microbalance experiments and X-ray photoelectron spectroscopy, we found that all studied iridium complexes form an iridium deposit on the electrode with binding energies ranging from 62.4 to 62.7 eV for the major Ir 4f7/2 species. These do not match the binding energies found for the parent complexes, which have a broader binding energy range from 61.7 to 62.7 eV and show a clear relationship to the electronegativity induced by the ligands. Moreover, all catalysts performed the electrochemical water oxidation in the same order of magnitude as the maximum currents ranged from 0.2 to 0.6 mA cm–2 once more without clear structure–activity relationships. In addition, by employing 1H NMR spectroscopy we found evidence for Cp* breakdown products such as acetate. Electrodeposited iridium oxide from ligand free [Ir(OH)6]2– or a colloidal iridium oxide nanoparticles solution produces currents almost 2 orders of magnitude higher with a maximum current of 11 mA cm–2. Also, this deposited material contains, apart from an Ir 4f7/2 species at 62.4 eV, an Ir species at 63.6 eV, which is not observed for any deposit formed by the molecular complexes. Thus, the electrodeposited material of the complexes cannot be directly linked to bulk iridium oxide. Small IrOx clusters containing few Ir atoms with partially incorporated ligand residues are the most likely option for the catalytically active electrodeposit. Our results emphasize that structure–activity relationships obtained with sacrificial oxidants do not necessarily translate to electrochemical conditions. Furthermore, other factors, such as electrodeposition and catalyst degradation, play a major role in the electrochemically driven water oxidation and should thus be considered when optimizing molecular catalysts.
中文翻译:

配体在铱介导的电催化水氧化中的影响
电化学水氧化是电解器的瓶颈,因为即使最好的催化剂铱和钌氧化物也必须在明显的超电势下运行。以前,发现羟基在一系列吡啶甲酸羟基配体上的位置会显着影响分子铱催化剂在牺牲性氧化剂驱动的水氧化中的活性。在这项研究中,这些催化剂在电化学条件下进行了测试,并在完全相同的条件下与其他几种已知的分子铱催化剂进行了对比。这使我们可以直接比较这些催化剂,并观察在电化学条件下结构-活性关系是否占优势。使用电化学石英晶体微量天平实验和X射线光电子能谱,7/2种。这些与母体复合物的结合能不匹配,后者的结合能范围在61.7至62.7 eV之间,与配体诱导的电负性表现出明显的关系。此外,所有催化剂都以相同的数量级执行了电化学水氧化反应,最大电流再次介于0.2至0.6 mA cm –2之间,而没有明确的结构-活性关系。此外,通过1 H NMR光谱,我们发现了Cp *分解产物(如乙酸盐)的证据。无配体[Ir(OH)6 ] 2的电沉积氧化铱或胶态氧化铱纳米粒子溶液产生的电流高出近2个数量级,最大电流为11 mA cm –2。另外,除了62.4eV处的Ir 4f 7/2物种外,该沉积材料还包含63.6eV处的Ir物种,对于由分子络合物形成的任何沉积物均未观察到。因此,络合物的电沉积材料不能直接与本体氧化铱连接。小IrO x含有少量掺有部分配体残基的Ir原子簇是催化活性电沉积的最可能选择。我们的结果强调,用牺牲性氧化剂获得的结构活性关系不一定转化为电化学条件。此外,其他因素,例如电沉积和催化剂降解,在电化学驱动的水氧化中起主要作用,因此在优化分子催化剂时应考虑这些因素。
更新日期:2020-03-26
中文翻译:

配体在铱介导的电催化水氧化中的影响
电化学水氧化是电解器的瓶颈,因为即使最好的催化剂铱和钌氧化物也必须在明显的超电势下运行。以前,发现羟基在一系列吡啶甲酸羟基配体上的位置会显着影响分子铱催化剂在牺牲性氧化剂驱动的水氧化中的活性。在这项研究中,这些催化剂在电化学条件下进行了测试,并在完全相同的条件下与其他几种已知的分子铱催化剂进行了对比。这使我们可以直接比较这些催化剂,并观察在电化学条件下结构-活性关系是否占优势。使用电化学石英晶体微量天平实验和X射线光电子能谱,7/2种。这些与母体复合物的结合能不匹配,后者的结合能范围在61.7至62.7 eV之间,与配体诱导的电负性表现出明显的关系。此外,所有催化剂都以相同的数量级执行了电化学水氧化反应,最大电流再次介于0.2至0.6 mA cm –2之间,而没有明确的结构-活性关系。此外,通过1 H NMR光谱,我们发现了Cp *分解产物(如乙酸盐)的证据。无配体[Ir(OH)6 ] 2的电沉积氧化铱或胶态氧化铱纳米粒子溶液产生的电流高出近2个数量级,最大电流为11 mA cm –2。另外,除了62.4eV处的Ir 4f 7/2物种外,该沉积材料还包含63.6eV处的Ir物种,对于由分子络合物形成的任何沉积物均未观察到。因此,络合物的电沉积材料不能直接与本体氧化铱连接。小IrO x含有少量掺有部分配体残基的Ir原子簇是催化活性电沉积的最可能选择。我们的结果强调,用牺牲性氧化剂获得的结构活性关系不一定转化为电化学条件。此外,其他因素,例如电沉积和催化剂降解,在电化学驱动的水氧化中起主要作用,因此在优化分子催化剂时应考虑这些因素。


























 京公网安备 11010802027423号
京公网安备 11010802027423号