当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
pH-Induced Changes in Polypeptide Conformation: Force-Field Comparison with Experimental Validation
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2020-03-26 , DOI: 10.1021/acs.jpcb.0c01475 Piotr Batys 1 , Maria Morga 1 , Piotr Bonarek 2 , Maria Sammalkorpi
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2020-03-26 , DOI: 10.1021/acs.jpcb.0c01475 Piotr Batys 1 , Maria Morga 1 , Piotr Bonarek 2 , Maria Sammalkorpi
Affiliation
|
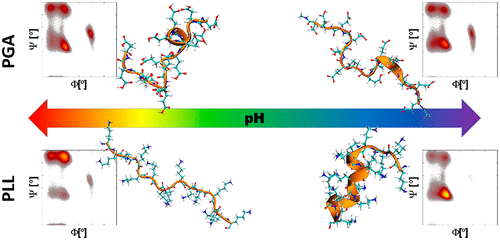
Microsecond-long all-atom molecular dynamics (MD) simulations, circular dichroism, laser Doppler velocimetry, and dynamic light-scattering techniques have been used to investigate pH-induced changes in the secondary structure, charge, and conformation of poly l-lysine (PLL) and poly l-glutamic acid (PGA). The employed combination of the experimental methods reveals for both PLL and PGA a narrow pH range at which they are charged enough to form stable colloidal suspensions, maintaining their α-helix content above 60%; an elevated charge state of the peptides required for colloidal stability promotes the peptide solvation as a random coil. To obtain a more microscopic view on the conformations and to verify the modeling performance, peptide secondary structure and conformations rising in MD simulations are also examined using three different force fields, i.e., OPLS-AA, CHARMM27, and AMBER99SB*-ILDNP. Ramachandran plots reveal that in the examined setup the α-helix content is systematically overestimated in CHARMM27, while OPLS-AA overestimates the β-sheet fraction at lower ionization degrees. At high ionization degrees, the OPLS-AA force-field-predicted secondary structure fractions match the experimentally measured distribution most closely. However, the pH-induced changes in PLL and PGA secondary structure are reasonably captured only by the AMBER99SB*-ILDNP force field, with the exception of the fully charged PGA in which the α-helix content is overestimated. The comparison to simulations results shows that the examined force fields involve significant deviations in their predictions for charged homopolypeptides. The detailed mapping of secondary structure dependency on pH for the polypeptides, especially finding the stable colloidal α-helical regime for both examined peptides, has significant potential for practical applications of the charged homopolypeptides. The findings raise attention especially to the pH fine tuning as an underappreciated control factor in surface modification and self-assembly.
中文翻译:

pH诱导的多肽构型变化:力场比较与实验验证
微秒长的全原子分子动力学(MD)模拟,圆二色性,激光多普勒测速仪和动态光散射技术已用于研究pH诱导的聚l-赖氨酸二级结构,电荷和构象的变化( PLL)和poly l-谷氨酸(PGA)。实验方法的结合使用为PLL和PGA揭示了一个狭窄的pH范围,在该pH范围内,它们的电荷足以形成稳定的胶体悬浮液,并保持其α-螺旋含量高于60%。胶体稳定性所需的肽带电状态的升高促进了作为无规卷曲的肽的溶剂化。为了获得有关构象的更微观视图并验证建模性能,还使用三个不同的力场(即OPLS-AA,CHARMM27和AMBER99SB * -ILDNP)检查了MD模拟中上升的肽二级结构和构象。Ramachandran图显示,在所检查的设置中,CHARMM27中的α-螺旋含量被系统地高估了,而OPLS-AA在较低的电离度下高估了β-折叠部分。在高电离度下 OPLS-AA力场预测的二级结构分数最匹配实验测量的分布。但是,只有AMBER99SB * -ILDNP力场才能合理地捕获pH引起的PLL和PGA二级结构的变化,除了充满电的PGA(其中α螺旋含量被高估)以外。与模拟结果的比较表明,所检查的力场在带电均多肽的预测中涉及显着偏差。多肽的二级结构依赖于pH的详细映射,特别是找到两种被检测肽的稳定胶体α-螺旋结构,对于带电荷的同型多肽的实际应用具有巨大的潜力。
更新日期:2020-03-27
中文翻译:

pH诱导的多肽构型变化:力场比较与实验验证
微秒长的全原子分子动力学(MD)模拟,圆二色性,激光多普勒测速仪和动态光散射技术已用于研究pH诱导的聚l-赖氨酸二级结构,电荷和构象的变化( PLL)和poly l-谷氨酸(PGA)。实验方法的结合使用为PLL和PGA揭示了一个狭窄的pH范围,在该pH范围内,它们的电荷足以形成稳定的胶体悬浮液,并保持其α-螺旋含量高于60%。胶体稳定性所需的肽带电状态的升高促进了作为无规卷曲的肽的溶剂化。为了获得有关构象的更微观视图并验证建模性能,还使用三个不同的力场(即OPLS-AA,CHARMM27和AMBER99SB * -ILDNP)检查了MD模拟中上升的肽二级结构和构象。Ramachandran图显示,在所检查的设置中,CHARMM27中的α-螺旋含量被系统地高估了,而OPLS-AA在较低的电离度下高估了β-折叠部分。在高电离度下 OPLS-AA力场预测的二级结构分数最匹配实验测量的分布。但是,只有AMBER99SB * -ILDNP力场才能合理地捕获pH引起的PLL和PGA二级结构的变化,除了充满电的PGA(其中α螺旋含量被高估)以外。与模拟结果的比较表明,所检查的力场在带电均多肽的预测中涉及显着偏差。多肽的二级结构依赖于pH的详细映射,特别是找到两种被检测肽的稳定胶体α-螺旋结构,对于带电荷的同型多肽的实际应用具有巨大的潜力。


























 京公网安备 11010802027423号
京公网安备 11010802027423号