当前位置:
X-MOL 学术
›
Macromolecules
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Stereoselective Copolymerization of Styrene with para-Substituted Styrenes Catalyzed by Half-Titanocenes: An Experimental and Computational Study
Macromolecules ( IF 5.5 ) Pub Date : 2020-03-17 , DOI: 10.1021/acs.macromol.9b02668 Chiara Costabile 1 , Annaluisa Mariconda 2 , Marco Sirignano 1 , Pasquale Longo 1
Macromolecules ( IF 5.5 ) Pub Date : 2020-03-17 , DOI: 10.1021/acs.macromol.9b02668 Chiara Costabile 1 , Annaluisa Mariconda 2 , Marco Sirignano 1 , Pasquale Longo 1
Affiliation
|
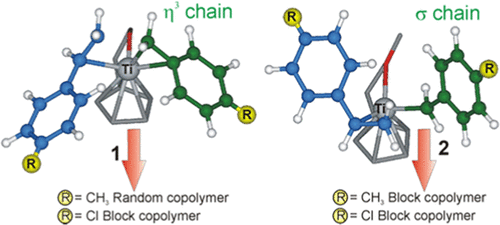
Copolymerization of styrene (S) with p-methylstyrene (PMS) and p-chlorostyrene (PCS), catalyzed by half-titanocenes (CpCH2CH2O)TiCl2 (1) and (CpCH2CH2OCH3)TiCl3 (2) activated by methylaluminoxane (MAO), was investigated from both experimental and computational points of view. The reactivity ratios and syndioselectivity of the reaction are affected by the styrene derivative and the catalytic system, leading to the formation of block or random copolymers. Using both catalytic systems, styrene, p-methylstyrene, and p-chlorostyrene show the same reactivity trend (PMS > S > PCS) already reported in the presence of the CpTiCl3/MAO catalyst and in the syndiotactic and isotactic polymerization of styrene derivatives with other Ti-based catalysts. According to density functional theory (DFT) calculations, extended also to the CpTiCl3/MAO catalyst, the reactivity of the employed comonomers is mainly due to the different electron-donating character of monomer para-substituents. As for stereoselectivity, (1) and (2) lead to syndiotactic copolymer sequences with PMS and S, whereas a loss of selectivity is observed in the poly-p-chlorostyrene homosequences of PCS/S copolymers. According to DFT studies, this lack of selectivity can be attributed to the presence of a σ terminal growing chain in the insertion transition states when PCS is involved in the polymerization.
中文翻译:

半钛茂金属催化的苯乙烯与对位取代的苯乙烯的立体选择性共聚:实验和计算研究
半钛茂金属(CpCH 2 CH 2 O)TiCl 2(1)和(CpCH 2 CH 2 OCH 3)TiCl 3((Sp )与对甲基苯乙烯(PMS)和对氯苯乙烯(PCS)的共聚合从实验和计算的角度研究了由甲基铝氧烷(MAO)活化的2)。苯乙烯衍生物和催化体系影响反应的反应率和间同选择性,导致形成嵌段或无规共聚物。使用两种催化体系,苯乙烯,对甲基苯乙烯和对苯二甲酸在CpTiCl 3 / MAO催化剂的存在下以及苯乙烯衍生物与其他Ti基催化剂的间同和等规聚合中,-氯苯乙烯显示出相同的反应性趋势(PMS> S> PCS)。根据密度泛函理论(DFT)计算,还扩展到CpTiCl 3 / MAO催化剂,所用共聚单体的反应性主要是由于单体对位取代基的供电子特性不同。至于立体选择性,(1)和(2)导致与PMS和S间同立构共聚物的序列,而选择性的损失在聚观察pPCS / S共聚物的-氯苯乙烯均聚物序列。根据DFT研究,当PCS参与聚合反应时,这种选择性的缺乏可归因于在插入过渡态中存在σ末端增长链。
更新日期:2020-04-24
中文翻译:

半钛茂金属催化的苯乙烯与对位取代的苯乙烯的立体选择性共聚:实验和计算研究
半钛茂金属(CpCH 2 CH 2 O)TiCl 2(1)和(CpCH 2 CH 2 OCH 3)TiCl 3((Sp )与对甲基苯乙烯(PMS)和对氯苯乙烯(PCS)的共聚合从实验和计算的角度研究了由甲基铝氧烷(MAO)活化的2)。苯乙烯衍生物和催化体系影响反应的反应率和间同选择性,导致形成嵌段或无规共聚物。使用两种催化体系,苯乙烯,对甲基苯乙烯和对苯二甲酸在CpTiCl 3 / MAO催化剂的存在下以及苯乙烯衍生物与其他Ti基催化剂的间同和等规聚合中,-氯苯乙烯显示出相同的反应性趋势(PMS> S> PCS)。根据密度泛函理论(DFT)计算,还扩展到CpTiCl 3 / MAO催化剂,所用共聚单体的反应性主要是由于单体对位取代基的供电子特性不同。至于立体选择性,(1)和(2)导致与PMS和S间同立构共聚物的序列,而选择性的损失在聚观察pPCS / S共聚物的-氯苯乙烯均聚物序列。根据DFT研究,当PCS参与聚合反应时,这种选择性的缺乏可归因于在插入过渡态中存在σ末端增长链。



























 京公网安备 11010802027423号
京公网安备 11010802027423号