当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Biosynthesis of a New Benzazepine Alkaloid Nanangelenin A from Aspergillus nanangensis Involves an Unusual L-Kynurenine-Incorporating NRPS Catalyzing Regioselective Lactamization
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2020-03-17 , DOI: 10.1021/jacs.0c01605 Hang Li , Cameron L. M. Gilchrist , Chin-Soon Phan , Heather J. Lacey 1 , Daniel Vuong 1 , Stephen A. Moggach , Ernest Lacey 1, 2 , Andrew M. Piggott 2 , Yit-Heng Chooi
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2020-03-17 , DOI: 10.1021/jacs.0c01605 Hang Li , Cameron L. M. Gilchrist , Chin-Soon Phan , Heather J. Lacey 1 , Daniel Vuong 1 , Stephen A. Moggach , Ernest Lacey 1, 2 , Andrew M. Piggott 2 , Yit-Heng Chooi
Affiliation
|
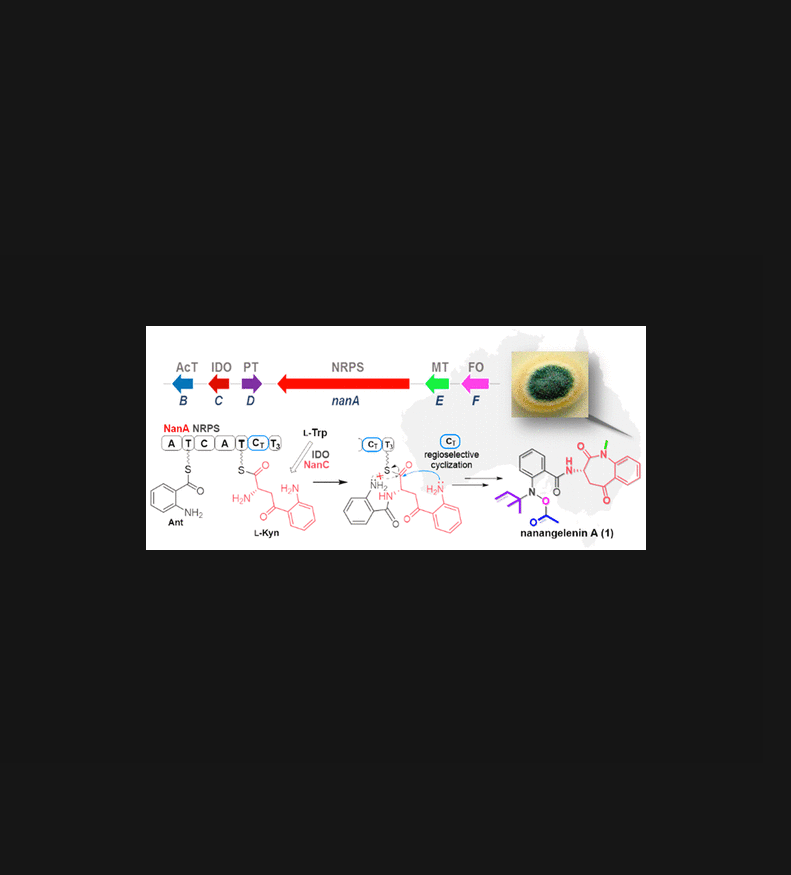
1-Benzazepine is a pharmaceutically important scaffold but is rare among natural products. Nanangelenin A (1), containing an unprecedented 3,4-dihydro-1-benzazepine-2,5-dione-N-prenyl-N-acetoxy-anthranilamide scaffold, was isolated from a novel species of Australian fungus, Aspergillus nanangensis. Genomic and retrobiosynthetic analyses identified a putative nonribosomal peptide synthetase (NRPS) gene cluster (nan). The detailed biosynthetic pathway to 1 was established by heterologous pathway reconstitution in A. nidulans, which led to biosynthesis of intermediates nanagelenin B-F (2-5 and 7). We demonstrated that the NRPS NanA incorporates anthranilic acid (Ant) and L-kynurenine (L-Kyn), which is supplied by a dedicated indoleamine-2,3-dioxygenase NanC encoded in the gene cluster. Using heterologous in vivo assays and mutagenesis, we demonstrated that the C-terminal condensation (CT) and thiolation (T3) domains of NanA are responsible for the regioselective cyclization of the tethered Ant-L-Kyn dipeptide to form the unusual benzazepine scaffold in 1. We also showed that NanA-CT catalyzes the regioselective cyclization of a surrogate synthetic substrate, Ant-L-Kyn-N-acetylcysteamine, to give the benzazepine scaffold, while spontaneous cyclization of the dipeptide yielded the alternative kinetically favored benzodiazepine scaffold. The discovery of 1 and the characterization of NanA have expanded the chemical and functional diversities of fungal NRPSs.
中文翻译:

从南南曲霉中生物合成一种新的苯并氮杂生物碱 Nanangelenin A 涉及一种不寻常的 L-犬尿氨酸结合 NRPS 催化区域选择性内酰胺化
1-苯扎西平是一种重要的药学支架,但在天然产物中很少见。Nanangelenin A (1) 含有前所未有的 3,4-dihydro-1-benzazepine-2,5-dione-N-prenyl-N-acetoxy-anthranilamide 支架,是从澳大利亚真菌的一种新物种南南曲霉中分离出来的。基因组和逆生物合成分析确定了一个假定的非核糖体肽合成酶 (NRPS) 基因簇 (nan)。详细的生物合成途径 1 是通过构巢曲霉中的异源途径重建建立的,这导致中间体 nanagelenin BF(2-5 和 7)的生物合成。我们证明了 NRPS NanA 结合了邻氨基苯甲酸 (Ant) 和 L-犬尿氨酸 (L-Kyn),它由基因簇中编码的专用吲哚胺-2,3-双加氧酶 NanC 提供。使用异源体内试验和诱变,我们证明了 NanA 的 C 端缩合 (CT) 和硫醇化 (T3) 结构域负责连接的 Ant-L-Kyn 二肽的区域选择性环化,以在 1 中形成不寻常的苯并氮杂支架。我们还表明 NanA-CT催化替代合成底物 Ant-L-Kyn-N-乙酰半胱胺的区域选择性环化,得到苯并氮杂支架,而二肽的自发环化产生了另一种动力学有利的苯二氮卓支架。1 的发现和 NanA 的表征扩展了真菌 NRPS 的化学和功能多样性。我们还表明,NanA-CT 催化替代合成底物 Ant-L-Kyn-N-乙酰半胱胺的区域选择性环化,得到苯并氮杂支架,而二肽的自发环化产生了另一种动力学有利的苯二氮卓支架。1 的发现和 NanA 的表征扩展了真菌 NRPS 的化学和功能多样性。我们还表明,NanA-CT 催化替代合成底物 Ant-L-Kyn-N-乙酰半胱胺的区域选择性环化,得到苯并氮杂支架,而二肽的自发环化产生了另一种动力学有利的苯二氮卓支架。1 的发现和 NanA 的表征扩展了真菌 NRPS 的化学和功能多样性。
更新日期:2020-03-17
中文翻译:

从南南曲霉中生物合成一种新的苯并氮杂生物碱 Nanangelenin A 涉及一种不寻常的 L-犬尿氨酸结合 NRPS 催化区域选择性内酰胺化
1-苯扎西平是一种重要的药学支架,但在天然产物中很少见。Nanangelenin A (1) 含有前所未有的 3,4-dihydro-1-benzazepine-2,5-dione-N-prenyl-N-acetoxy-anthranilamide 支架,是从澳大利亚真菌的一种新物种南南曲霉中分离出来的。基因组和逆生物合成分析确定了一个假定的非核糖体肽合成酶 (NRPS) 基因簇 (nan)。详细的生物合成途径 1 是通过构巢曲霉中的异源途径重建建立的,这导致中间体 nanagelenin BF(2-5 和 7)的生物合成。我们证明了 NRPS NanA 结合了邻氨基苯甲酸 (Ant) 和 L-犬尿氨酸 (L-Kyn),它由基因簇中编码的专用吲哚胺-2,3-双加氧酶 NanC 提供。使用异源体内试验和诱变,我们证明了 NanA 的 C 端缩合 (CT) 和硫醇化 (T3) 结构域负责连接的 Ant-L-Kyn 二肽的区域选择性环化,以在 1 中形成不寻常的苯并氮杂支架。我们还表明 NanA-CT催化替代合成底物 Ant-L-Kyn-N-乙酰半胱胺的区域选择性环化,得到苯并氮杂支架,而二肽的自发环化产生了另一种动力学有利的苯二氮卓支架。1 的发现和 NanA 的表征扩展了真菌 NRPS 的化学和功能多样性。我们还表明,NanA-CT 催化替代合成底物 Ant-L-Kyn-N-乙酰半胱胺的区域选择性环化,得到苯并氮杂支架,而二肽的自发环化产生了另一种动力学有利的苯二氮卓支架。1 的发现和 NanA 的表征扩展了真菌 NRPS 的化学和功能多样性。我们还表明,NanA-CT 催化替代合成底物 Ant-L-Kyn-N-乙酰半胱胺的区域选择性环化,得到苯并氮杂支架,而二肽的自发环化产生了另一种动力学有利的苯二氮卓支架。1 的发现和 NanA 的表征扩展了真菌 NRPS 的化学和功能多样性。


























 京公网安备 11010802027423号
京公网安备 11010802027423号