当前位置:
X-MOL 学术
›
Arch. Pharm.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Structure-based design, synthesis, and evaluation of Bcl-2/Mcl-1 dual inhibitors
Archiv der Pharmazie ( IF 5.1 ) Pub Date : 2020-03-16 , DOI: 10.1002/ardp.202000005 Junjie Zhu 1 , Ziqian Wang 2 , Zongwei Guo 3 , Xiaodong Zhang 1 , Ting Song 1 , Yafei Guo 3 , Tong Ji 1 , Zhichao Zhang 1
Archiv der Pharmazie ( IF 5.1 ) Pub Date : 2020-03-16 , DOI: 10.1002/ardp.202000005 Junjie Zhu 1 , Ziqian Wang 2 , Zongwei Guo 3 , Xiaodong Zhang 1 , Ting Song 1 , Yafei Guo 3 , Tong Ji 1 , Zhichao Zhang 1
Affiliation
|
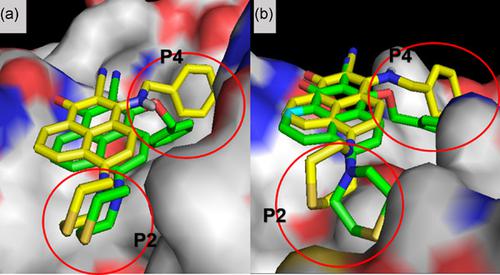
Based on our previously reported Bcl‐2/Mcl‐1 dual inhibitor 4‐thiomorpholinyl‐2‐cyano‐3‐amidinophenalenone (A1) that simultaneously occupies the p2 and p4 hydrophobic pockets of Bcl‐2 and Mcl‐1, we optimized molecules with different bond angles of the groups extending to the p4 pocket and bulky hydrophobic groups to explore p2. Research on structure–activity relationship resulted in a new derivative B4 that is capable of occupying both the p2 and p4 more deeply and completely than A1, with Ki values determined by fluorescence polarization assay (FPAs) improving to 0.31 μM for Bcl‐2 and 0.16 μM for Mcl‐1. Furthermore, B4 exhibited selective lethality on cancer cells over normal cells. It showed stronger apoptosis induction than (–)‐gossypol on a Bcl‐2/Mcl‐1‐dependent cancer cell line and killed an Mcl‐1‐dependent cell line which is resistant to ABT‐199 treatment.
中文翻译:

Bcl-2/Mcl-1双重抑制剂的基于结构的设计、合成和评价
基于我们之前报道的 Bcl-2/Mcl-1 双重抑制剂 4-thiomorpholinyl-2-cyano-3-amidinophenalenone (A1),它同时占据 Bcl-2 和 Mcl-1 的 p2 和 p4 疏水口袋,我们优化了分子延伸到 p4 口袋的基团和庞大的疏水基团的不同键角以探索 p2。构效关系的研究产生了一种新的衍生物 B4,它能够比 A1 更深更完整地占据 p2 和 p4,通过荧光偏振测定 (FPA) 测定的 Ki 值对于 Bcl-2 和 0.16 提高到 0.31 μM Mcl-1 的 μM。此外,与正常细胞相比,B4 对癌细胞的选择性杀伤力。
更新日期:2020-03-16
中文翻译:

Bcl-2/Mcl-1双重抑制剂的基于结构的设计、合成和评价
基于我们之前报道的 Bcl-2/Mcl-1 双重抑制剂 4-thiomorpholinyl-2-cyano-3-amidinophenalenone (A1),它同时占据 Bcl-2 和 Mcl-1 的 p2 和 p4 疏水口袋,我们优化了分子延伸到 p4 口袋的基团和庞大的疏水基团的不同键角以探索 p2。构效关系的研究产生了一种新的衍生物 B4,它能够比 A1 更深更完整地占据 p2 和 p4,通过荧光偏振测定 (FPA) 测定的 Ki 值对于 Bcl-2 和 0.16 提高到 0.31 μM Mcl-1 的 μM。此外,与正常细胞相比,B4 对癌细胞的选择性杀伤力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号