当前位置:
X-MOL 学术
›
Water Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Formation and control of bromate in sulfate radical-based oxidation processes for the treatment of waters containing bromide: A critical review.
Water Research ( IF 12.8 ) Pub Date : 2020-03-16 , DOI: 10.1016/j.watres.2020.115725 Chaoting Guan 1 , Jin Jiang 1 , Suyan Pang 2 , Yang Zhou 3 , Yuan Gao 3 , Juan Li 1 , Zhen Wang 1
Water Research ( IF 12.8 ) Pub Date : 2020-03-16 , DOI: 10.1016/j.watres.2020.115725 Chaoting Guan 1 , Jin Jiang 1 , Suyan Pang 2 , Yang Zhou 3 , Yuan Gao 3 , Juan Li 1 , Zhen Wang 1
Affiliation
|
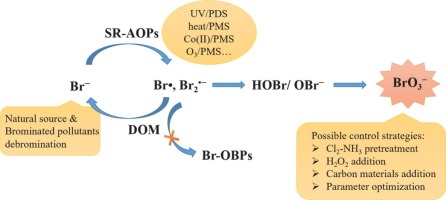
Sulfate radical-based advanced oxidation processes (SR-AOPs) show a good prospect for effective elimination of organic contaminants in water due to the powerful oxidation capability and good adaptability of sulfate radical (SO4•-). However, great concerns have been raised on occurrence of the carcinogenic byproduct bromate (BrO3-) in SR-AOPs. The present article aims to provide a critical review on BrO3- formation during bromine (Br)-containing water oxidation by various SR-AOPs. Potential reaction mechanisms are elaborated, mainly involving the sequential oxidation of bromide (Br-) by SO4•- to Br-containing radicals (e.g., bromine atom (Br•)) and then to hypobromous acid/hypobromite (HOBr/OBr-), which acts as the requisite intermediate for BrO3- formation. Some key influencing factors on BrO3- formation are discussed. Particularly, dissolved organic matter (DOM) as a component ubiquitously present in aquatic environments shows a significant suppression effect on BrO3- formation, primarily attributed to the reduction of Br• by DOM to Br-. The reaction of Br• with DOM can hardly produce organic brominated byproducts, while their formation is mainly due to the bromination of HOBr/OBr- generated through nonradical pathways such as the direct reaction of Br- with oxidants (e.g., peroxymonosulfate (PMS)) or other reactive species derived from catalytic activators (e.g., Co(III) in the Co(II)/PMS process). The debromination of brominated pollutants during their oxidation by SO4•- results in the release of Br-, which, however, is not further transformed to BrO3- until coexisting organic matters are mineralized nearly completely. Furthermore, possible strategies for control of BrO3- formation in SR-AOPs as well as the future research needs are proposed.
中文翻译:

硫酸根自由基氧化工艺中溴酸盐的形成和控制,该工艺用于处理含溴化物的水:一项重要的综述。
基于硫酸根的高级氧化工艺(SR-AOP)由于具有强大的氧化能力和良好的硫酸根(SO4•-)适应性,因此有望有效消除水中的有机污染物。但是,人们对在SR-AOP中产生致癌副产物溴酸盐(BrO3-)引起了极大的关注。本文旨在就各种SR-AOP在含溴(Br)的水氧化过程中对BrO3-的形成提供重要的综述。阐述了潜在的反应机理,主要涉及通过SO4•-将溴化物(Br-)依次氧化为含溴的自由基(例如溴原子(Br•)),然后再氧化为次溴酸/次溴酸盐(HOBr / OBr-),它是形成BrO3-的必要中间体。讨论了影响BrO3-形成的一些关键因素。尤其,在水生环境中普遍存在的溶解有机物(DOM)表现出对BrO3-形成的显着抑制作用,这主要归因于DOM将Br•还原为Br-。Br•与DOM的反应几乎不会产生有机溴化副产物,而它们的形成主要是由于通过非自由基途径(例如Br-与氧化剂(例如过一硫酸氢盐(PMS))的直接反应)生成的HOBr / OBr-的溴化。或其他源自催化活化剂的反应性物种(例如,Co(II)/ PMS工艺中的Co(III))。溴污染物在被SO4•-氧化过程中脱溴会导致Br-的释放,但是直到并存的有机物几乎被完全矿化之前,Br-不会进一步转化为BrO3-。此外,
更新日期:2020-03-16
中文翻译:

硫酸根自由基氧化工艺中溴酸盐的形成和控制,该工艺用于处理含溴化物的水:一项重要的综述。
基于硫酸根的高级氧化工艺(SR-AOP)由于具有强大的氧化能力和良好的硫酸根(SO4•-)适应性,因此有望有效消除水中的有机污染物。但是,人们对在SR-AOP中产生致癌副产物溴酸盐(BrO3-)引起了极大的关注。本文旨在就各种SR-AOP在含溴(Br)的水氧化过程中对BrO3-的形成提供重要的综述。阐述了潜在的反应机理,主要涉及通过SO4•-将溴化物(Br-)依次氧化为含溴的自由基(例如溴原子(Br•)),然后再氧化为次溴酸/次溴酸盐(HOBr / OBr-),它是形成BrO3-的必要中间体。讨论了影响BrO3-形成的一些关键因素。尤其,在水生环境中普遍存在的溶解有机物(DOM)表现出对BrO3-形成的显着抑制作用,这主要归因于DOM将Br•还原为Br-。Br•与DOM的反应几乎不会产生有机溴化副产物,而它们的形成主要是由于通过非自由基途径(例如Br-与氧化剂(例如过一硫酸氢盐(PMS))的直接反应)生成的HOBr / OBr-的溴化。或其他源自催化活化剂的反应性物种(例如,Co(II)/ PMS工艺中的Co(III))。溴污染物在被SO4•-氧化过程中脱溴会导致Br-的释放,但是直到并存的有机物几乎被完全矿化之前,Br-不会进一步转化为BrO3-。此外,



























 京公网安备 11010802027423号
京公网安备 11010802027423号