当前位置:
X-MOL 学术
›
Mater. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Influence of dicationic quaternary ammonium gemini surfactant system on metal-amino acid complex-ninhydrin reaction
Materials Chemistry and Physics ( IF 4.6 ) Pub Date : 2020-07-01 , DOI: 10.1016/j.matchemphys.2020.122926 Dileep Kumar , Malik Abdul Rub
Materials Chemistry and Physics ( IF 4.6 ) Pub Date : 2020-07-01 , DOI: 10.1016/j.matchemphys.2020.122926 Dileep Kumar , Malik Abdul Rub
|
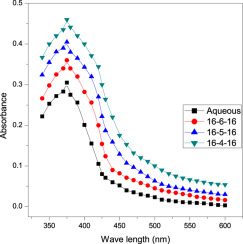
Abstract In the current study, we have elucidated the influence of dicationic quaternary ammonium geminis system on metal-amino acid [Ni(II)-his]+ complex-ninhydrin reaction with the help of UV–vis spectrophometer at 343 K and pH 5.0. Under varying experimental conditions, rate constant values, kψ, were determined using a computer-based program. Quaternary ammonium gemini systems (rate constant values of 16-6-16, 16-5-16 and 16-4-16 at 30 × 10−5 mol dm−3 are 5.5 × 10−5 s−1, 6.5 × 10−5 s−1 and 7.5 × 10−5 s−1, respectively) are detected more superior compared to aqueous system (rate constant in aqueous is 1.7 × 10−5 s−1). Study was catalyzed and accelerated by gemini surfactants (even though at concentrations below than their cmc values) compared to aqueous medium. Rate constant increases progressively on increasing [gemini] (region I, where [gemini] is smaller than their cmc) and levelling-off regions attain (region II, at [gemini] up to 400 × 10−5 mol dm−3). Afterward, gemini provides a region III of increasing kψ at higher concentration (region III, beyond 400 × 10−5 mol dm−3). Experimental results acquired in dicationic quaternary ammonium gemini surfactant system are deduced quantitatively by kinetic pseudo-phase model. For determination of cmc of geminis having a different methylene spacer chain length (s = 4, 5, 6), the specific conductance at varied [16-6-16], [16-5-16] and [16-4-16] (i.e., water and water + ninhydrin + [Ni(II)-his]+) were 0.043 × 10−3 mol dm−3 at 303 K and 0.053 × 10−3 mol dm−3 at 343 K; 0.034 × 10−3 mol dm−3 at 303 K and 0.044 × 10−3 mol dm−3 at 343 K; 0.032 × 10−3 mol dm−3 at 303 K; 0.040 × 10−3 mol dm−3 at 343 K, respectively, recorded on a conductivity meter. Several activation parameters for 16-6-16, 16-5-16 and 16-4-16 (ΔH# = 45.0, 43.5 and 42.0 kJ mol−1; ΔS# 87.0, 87.6 and 88.3 JK-1; Ea = 47.8, 46.3 and 44.8 kJ mol−1) and binding parameters for 16-6-16, 16-5-16 and 16-4-16 (KX = 63.0, 58.0 and 54.0 mol−1 dm3; KY = 70.0, 66.0 and 62.0 mol−1 dm3) are also determined.
中文翻译:

双阳离子季铵双子表面活性剂体系对金属-氨基酸络合物-茚三酮反应的影响
摘要 在目前的研究中,我们在 343 K 和 pH 5.0 的紫外可见分光光度计的帮助下阐明了双阳离子季铵双子体系对金属-氨基酸 [Ni(II)-his]+ 络合物-茚三酮反应的影响。在不同的实验条件下,使用基于计算机的程序确定速率常数值 kψ。季铵双子系统(16-6-16、16-5-16 和 16-4-16 在 30 × 10−5 mol dm−3 下的速率常数值为 5.5 × 10−5 s−1, 6.5 × 10−分别检测到 5 s-1 和 7.5 × 10-5 s-1)比水性体系更优越(水性体系中的速率常数为 1.7 × 10-5 s-1)。与水性介质相比,研究由双子表面活性剂(即使浓度低于其 cmc 值)催化和加速。速率常数随着 [gemini] 的增加(区域 I,其中 [gemini] 小于它们的 cmc) 并且达到平坦区域(区域 II,在 [gemini] 高达 400 × 10-5 mol dm-3)。之后,双子座提供了一个在较高浓度下增加 kψ 的区域 III(区域 III,超过 400 × 10-5 mol dm-3)。通过动力学假相模型定量推导出双阳离子季铵双子表面活性剂体系的实验结果。为了测定具有不同亚甲基间隔链长度 (s = 4, 5, 6) 的双子座的 cmc,在不同的 [16-6-16]、[16-5-16] 和 [16-4-16 ](即水和水 + 茚三酮 + [Ni(II)-his]+)在 303 K 时为 0.043 × 10-3 mol dm-3,在 343 K 时为 0.053 × 10-3 mol dm-3;0.034 × 10-3 mol dm-3 在 303 K 和 0.044 × 10-3 mol dm-3 在 343 K;0.032 × 10−3 mol dm−3 在 303 K;在 343 K 时分别为 0.040 × 10−3 mol dm−3,记录在电导仪上。16-6-16、16-5-16 和 16-4-16 的几个活化参数(ΔH# = 45.0、43.5 和 42.0 kJ mol−1;ΔS# 87.0、87.6 和 88.3 JK-1;Ea = 47.8, 46.3 和 44.8 kJ mol−1) 和 16-6-16、16-5-16 和 16-4-16 的结合参数(KX = 63.0、58.0 和 54.0 mol−1 dm3;KY = 70.0、66.0 和 62.0 mol −1 dm3) 也被确定。
更新日期:2020-07-01
中文翻译:

双阳离子季铵双子表面活性剂体系对金属-氨基酸络合物-茚三酮反应的影响
摘要 在目前的研究中,我们在 343 K 和 pH 5.0 的紫外可见分光光度计的帮助下阐明了双阳离子季铵双子体系对金属-氨基酸 [Ni(II)-his]+ 络合物-茚三酮反应的影响。在不同的实验条件下,使用基于计算机的程序确定速率常数值 kψ。季铵双子系统(16-6-16、16-5-16 和 16-4-16 在 30 × 10−5 mol dm−3 下的速率常数值为 5.5 × 10−5 s−1, 6.5 × 10−分别检测到 5 s-1 和 7.5 × 10-5 s-1)比水性体系更优越(水性体系中的速率常数为 1.7 × 10-5 s-1)。与水性介质相比,研究由双子表面活性剂(即使浓度低于其 cmc 值)催化和加速。速率常数随着 [gemini] 的增加(区域 I,其中 [gemini] 小于它们的 cmc) 并且达到平坦区域(区域 II,在 [gemini] 高达 400 × 10-5 mol dm-3)。之后,双子座提供了一个在较高浓度下增加 kψ 的区域 III(区域 III,超过 400 × 10-5 mol dm-3)。通过动力学假相模型定量推导出双阳离子季铵双子表面活性剂体系的实验结果。为了测定具有不同亚甲基间隔链长度 (s = 4, 5, 6) 的双子座的 cmc,在不同的 [16-6-16]、[16-5-16] 和 [16-4-16 ](即水和水 + 茚三酮 + [Ni(II)-his]+)在 303 K 时为 0.043 × 10-3 mol dm-3,在 343 K 时为 0.053 × 10-3 mol dm-3;0.034 × 10-3 mol dm-3 在 303 K 和 0.044 × 10-3 mol dm-3 在 343 K;0.032 × 10−3 mol dm−3 在 303 K;在 343 K 时分别为 0.040 × 10−3 mol dm−3,记录在电导仪上。16-6-16、16-5-16 和 16-4-16 的几个活化参数(ΔH# = 45.0、43.5 和 42.0 kJ mol−1;ΔS# 87.0、87.6 和 88.3 JK-1;Ea = 47.8, 46.3 和 44.8 kJ mol−1) 和 16-6-16、16-5-16 和 16-4-16 的结合参数(KX = 63.0、58.0 和 54.0 mol−1 dm3;KY = 70.0、66.0 和 62.0 mol −1 dm3) 也被确定。



























 京公网安备 11010802027423号
京公网安备 11010802027423号