当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Specific stabilization of promoter G-Quadruplex DNA by 2,6-disubstituted amidoanthracene-9,10-dione based dimeric distamycin analogues and their selective cancer cell cytotoxicity.
European Journal of Medicinal Chemistry ( IF 6.7 ) Pub Date : 2020-03-16 , DOI: 10.1016/j.ejmech.2020.112202 Soma Roy 1 , Asfa Ali 1 , Mohini Kamra 1 , Kalappa Muniyappa 2 , Santanu Bhattacharya 3
European Journal of Medicinal Chemistry ( IF 6.7 ) Pub Date : 2020-03-16 , DOI: 10.1016/j.ejmech.2020.112202 Soma Roy 1 , Asfa Ali 1 , Mohini Kamra 1 , Kalappa Muniyappa 2 , Santanu Bhattacharya 3
Affiliation
|
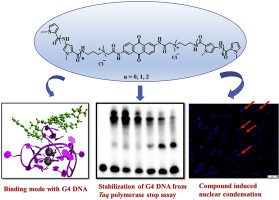
We have designed and synthesized anthraquinone containing compounds which have oligopyrrole side chains of varying lengths. These compounds stabilized the G-quadruplex DNA formed in the promoter regions of c-MYC oncogenes selectively over the duplex DNA. These observations were recorded using UV-vis spectroscopic titrations, fluorescence measurements and circular dichroism (CD) spectral titrations. The potency of the compounds to stabilize the G4 DNA has been shown from the thermal denaturation experiments. The compound interacts with c-MYC G-quadruplex DNA through stacking mode as obtained from ethidium bromide displacement assay, cyclic voltammetric titration, and docking experiments. Molecular modeling studies suggested that the stacking of the anthraquinone moiety over the G-tetrad of the G4 structures are responsible for the stability of such quadruplex secondary structure. Furthermore, polymerase stop assay also supported the formation of stable G4 structures in the presence of the above-mentioned compounds. The compounds have shown selective cancer cell (HeLa and HEK293T) cytotoxicity over normal cells (NIH3T3 and HDFa) under in vitro conditions as determined from MTT based cell viability assay. Apoptosis was found to be the mechanistic pathway underlying the cancer cell cytotoxicity as obtained from Annexin V-FITC and PI dual staining assay which was further substantiated by nuclear morphological changes as observed by AO/EB dual staining assay. Cellular morphological changes, as well as nuclear condensation and fragmentation upon treatment with these compounds, were observed under bright field and confocal microscopy.
中文翻译:

基于2,6-二取代的氨基蒽-9,10-二酮的二聚双霉素类似物对启动子G-Quadruplex DNA的特异性稳定作用及其选择性的癌细胞毒性。
我们已经设计并合成了含有蒽醌的化合物,这些化合物具有不同长度的寡吡咯侧链。这些化合物稳定了在c-MYC癌基因的启动子区域中形成的G-四链体DNA,选择性地超过了双链体DNA。使用紫外可见光谱滴定,荧光测量和圆二色性(CD)光谱滴定记录这些观察结果。从热变性实验已经显示出化合物稳定G4 DNA的效力。该化合物通过堆叠模式与c-MYC G-四链体DNA相互作用,该堆叠模式是从溴化乙锭置换试验,循环伏安滴定和对接实验中获得的。分子模型研究表明,蒽醌部分在G4结构的G-四联体上的堆积负责这种四链体二级结构的稳定性。此外,在上述化合物存在下,聚合酶终止试验也支持稳定的G4结构的形成。根据基于MTT的细胞生存力测定法确定的化合物,在体外条件下,该化合物对正常细胞(NIH3T3和HDFa)具有选择性的癌细胞(HeLa和HEK293T)细胞毒性。从膜联蛋白V-FITC和PI双重染色测定法中发现,凋亡是癌细胞细胞毒性的机制途径,其通过AO / EB双重染色测定法观察到的核形态变化进一步证实。细胞形态变化
更新日期:2020-03-16
中文翻译:

基于2,6-二取代的氨基蒽-9,10-二酮的二聚双霉素类似物对启动子G-Quadruplex DNA的特异性稳定作用及其选择性的癌细胞毒性。
我们已经设计并合成了含有蒽醌的化合物,这些化合物具有不同长度的寡吡咯侧链。这些化合物稳定了在c-MYC癌基因的启动子区域中形成的G-四链体DNA,选择性地超过了双链体DNA。使用紫外可见光谱滴定,荧光测量和圆二色性(CD)光谱滴定记录这些观察结果。从热变性实验已经显示出化合物稳定G4 DNA的效力。该化合物通过堆叠模式与c-MYC G-四链体DNA相互作用,该堆叠模式是从溴化乙锭置换试验,循环伏安滴定和对接实验中获得的。分子模型研究表明,蒽醌部分在G4结构的G-四联体上的堆积负责这种四链体二级结构的稳定性。此外,在上述化合物存在下,聚合酶终止试验也支持稳定的G4结构的形成。根据基于MTT的细胞生存力测定法确定的化合物,在体外条件下,该化合物对正常细胞(NIH3T3和HDFa)具有选择性的癌细胞(HeLa和HEK293T)细胞毒性。从膜联蛋白V-FITC和PI双重染色测定法中发现,凋亡是癌细胞细胞毒性的机制途径,其通过AO / EB双重染色测定法观察到的核形态变化进一步证实。细胞形态变化



























 京公网安备 11010802027423号
京公网安备 11010802027423号