当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis of chromenone, pyrimidinone, thiazoline, and quinolone derivatives as prospective antitumor agents
Journal of Heterocyclic Chemistry ( IF 2.4 ) Pub Date : 2020-03-14 , DOI: 10.1002/jhet.3948 Eman A. E. El‐Helw 1 , Azza A. El‐Badawy 1
Journal of Heterocyclic Chemistry ( IF 2.4 ) Pub Date : 2020-03-14 , DOI: 10.1002/jhet.3948 Eman A. E. El‐Helw 1 , Azza A. El‐Badawy 1
Affiliation
|
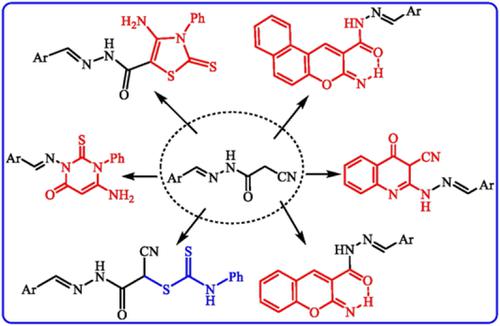
Hydrazide‐hydrazone namely, 2‐cyano‐N ′‐((1‐phenyl‐3‐[thiophen‐2‐yl]‐1H ‐pyrazol‐4‐yl)methylene)acetohydrazide (3) underwent a series of reactions with some chemical reagents to construct new biologically active N ‐heterocycles, for example, chromenone, benzochromenone, thiazoline, and quinolone derivatives. Treating the nitrile derivative 3 with 2,4‐dichlorobenzaldehyde and pyrazole aldehyde 1 afforded the corresponding condensed products. Some of the synthesized compounds were screened for their in vitro antitumor activities against two different human tumor cell lines including hepatocellular liver carcinoma (HepG2) and breast adenocarcinoma (MCF7) activities. Compound 3 was the most potent against the two tumors.
中文翻译:

色酮,嘧啶酮,噻唑啉和喹诺酮衍生物的合成作为预期的抗肿瘤药
酰肼‐,即2-氰基N '-((1-苯基-3- [噻吩-2-基] -1 H-吡唑-4-基)亚甲基)乙酰肼(3)进行了一系列反应,其中一些化学试剂以构建新的具有生物活性的N杂环,例如色酮,苯并色酮,噻唑啉和喹诺酮衍生物。用2,4-二氯苯甲醛和吡唑醛1处理腈衍生物3,得到相应的缩合物。筛选了一些合成的化合物对两种不同人类肿瘤细胞系的体外抗肿瘤活性,包括肝细胞肝癌(HepG2)和乳腺腺癌(MCF7)活性。复合3对两种肿瘤最有效。
更新日期:2020-03-14
中文翻译:

色酮,嘧啶酮,噻唑啉和喹诺酮衍生物的合成作为预期的抗肿瘤药
酰肼‐,即2-氰基N '-((1-苯基-3- [噻吩-2-基] -1 H-吡唑-4-基)亚甲基)乙酰肼(3)进行了一系列反应,其中一些化学试剂以构建新的具有生物活性的N杂环,例如色酮,苯并色酮,噻唑啉和喹诺酮衍生物。用2,4-二氯苯甲醛和吡唑醛1处理腈衍生物3,得到相应的缩合物。筛选了一些合成的化合物对两种不同人类肿瘤细胞系的体外抗肿瘤活性,包括肝细胞肝癌(HepG2)和乳腺腺癌(MCF7)活性。复合3对两种肿瘤最有效。


























 京公网安备 11010802027423号
京公网安备 11010802027423号