当前位置:
X-MOL 学术
›
J. Heterocycl. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis and antidiabetic activity of novel triazole derivatives containing amino acids
Journal of Heterocyclic Chemistry ( IF 2.4 ) Pub Date : 2020-03-12 , DOI: 10.1002/jhet.3951 Mounir A. A. Mohamed 1 , Omyma A. Abd Allah 1 , Adnan A. Bekhit 2, 3 , Asmaa M. Kadry 1 , Ahmed M. M. El‐Saghier 1
Journal of Heterocyclic Chemistry ( IF 2.4 ) Pub Date : 2020-03-12 , DOI: 10.1002/jhet.3951 Mounir A. A. Mohamed 1 , Omyma A. Abd Allah 1 , Adnan A. Bekhit 2, 3 , Asmaa M. Kadry 1 , Ahmed M. M. El‐Saghier 1
Affiliation
|
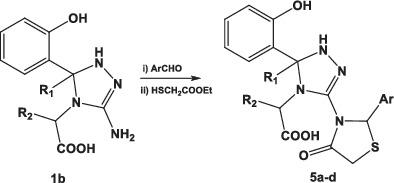
New series of triazole derivatives coupled with amino acids 1a‐h were obtained via multicomponent reaction of 2‐hydroxy benzaldehyde or 2‐hydroxy acetophenone with thiosemicarbazide and different amino acids. The obtained compounds were reacted with p ‐toluinesulfonyl chloride 2 to give the corresponding sulfonamides 3a‐h . Compound 1b was allowed to react with different aromatic aldehydes or cyclic ketone under alkaline conditions to afford the expected imino compounds 4a‐d and 6a‐c , respectively. These compounds were allowed to react with ethyl glycolate to yield the expected thiazolidinone derivatives 5a‐d or 7a‐c , respectively. Structures of the newly synthesized compounds were found to be in accordance with their elemental analyses and spectral data. The obtained compounds exhibited very prominent in vitro and in vivo antihyperglycemic effect at a dose of 40 mg/kg body weight compared to the standard drug gliclazide and control. The antidiabetic effect was investigated using oral glucose tolerance test in normal and non‐insulin‐dependent diabetes mellitus (NIDDM) in STZ‐rat model. Compounds 3a ‐h , 5b , 5c , 5d , 7a , 7b , and 7c showed significant activity in lowering blood glucose (more than 80%) compared to the NIDDM control.
中文翻译:

新型含氨基酸三唑衍生物的合成及抗糖尿病活性
通过2-羟基苯甲醛或2-羟基苯乙酮与硫代氨基脲和不同氨基酸的多组分反应,获得了与氨基酸1a-h偶联的新系列三唑衍生物。将获得的化合物与对甲苯胺磺酰氯2反应,得到相应的磺酰胺3a-h。使化合物1b在碱性条件下与不同的芳族醛或环状酮反应,分别得到预期的亚氨基化合物4a-d和6a-c。使这些化合物与乙醇酸乙酯反应,生成预期的噻唑烷酮衍生物5a-d或分别为7a-c。发现新合成的化合物的结构与其元素分析和光谱数据一致。与标准药物格列齐特和对照相比,所获得的化合物在40 mg / kg体重的剂量下具有非常突出的体外和体内降血糖作用。在正常和非胰岛素依赖型糖尿病(NIDDM)的STZ大鼠模型中,通过口服葡萄糖耐量试验研究了其抗糖尿病作用。化合物3a - h,5b,5c,5d,7a,7b和7c 与NIDDM对照相比,在降低血糖方面具有显着活性(超过80%)。
更新日期:2020-03-12
中文翻译:

新型含氨基酸三唑衍生物的合成及抗糖尿病活性
通过2-羟基苯甲醛或2-羟基苯乙酮与硫代氨基脲和不同氨基酸的多组分反应,获得了与氨基酸1a-h偶联的新系列三唑衍生物。将获得的化合物与对甲苯胺磺酰氯2反应,得到相应的磺酰胺3a-h。使化合物1b在碱性条件下与不同的芳族醛或环状酮反应,分别得到预期的亚氨基化合物4a-d和6a-c。使这些化合物与乙醇酸乙酯反应,生成预期的噻唑烷酮衍生物5a-d或分别为7a-c。发现新合成的化合物的结构与其元素分析和光谱数据一致。与标准药物格列齐特和对照相比,所获得的化合物在40 mg / kg体重的剂量下具有非常突出的体外和体内降血糖作用。在正常和非胰岛素依赖型糖尿病(NIDDM)的STZ大鼠模型中,通过口服葡萄糖耐量试验研究了其抗糖尿病作用。化合物3a - h,5b,5c,5d,7a,7b和7c 与NIDDM对照相比,在降低血糖方面具有显着活性(超过80%)。



























 京公网安备 11010802027423号
京公网安备 11010802027423号