当前位置:
X-MOL 学术
›
Biotechnol. Bioeng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Systems metabolic engineering of Bacillus subtilis for efficient biosynthesis of 5-methyltetrahydrofolate.
Biotechnology and Bioengineering ( IF 3.8 ) Pub Date : 2020-03-14 , DOI: 10.1002/bit.27332 Han Yang 1, 2 , Yanfeng Liu 1, 2 , Jianghua Li 1, 2 , Long Liu 1, 2 , Guocheng Du 1, 2 , Jian Chen 2, 3
Biotechnology and Bioengineering ( IF 3.8 ) Pub Date : 2020-03-14 , DOI: 10.1002/bit.27332 Han Yang 1, 2 , Yanfeng Liu 1, 2 , Jianghua Li 1, 2 , Long Liu 1, 2 , Guocheng Du 1, 2 , Jian Chen 2, 3
Affiliation
|
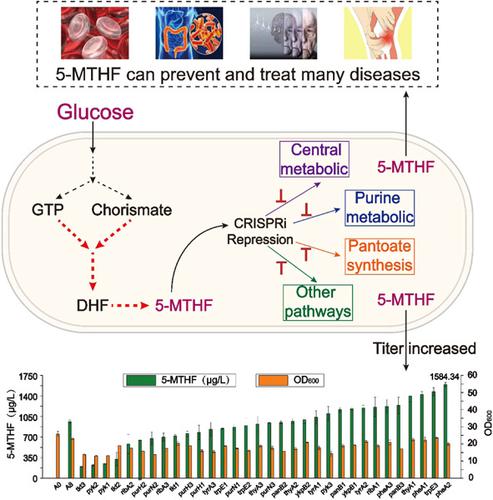
5‐Methyltetrahydrofolate (5‐MTHF) is the major form of folate in human plasma and is the only folate form that can penetrate the blood–brain barrier. It has been widely used for the prevention and treatment of various diseases. It is mainly produced by chemical synthesis. However, the low production rate cannot meet the increasing demand. In addition, chemical synthesis is potentially detrimental to the environment. Despite various microorganisms synthetizing 5‐MTHF, an efficient 5‐MTHF bioproduction approach is lacking because of the tight regulation of the 5‐MTHF pathway and limited metabolic flux toward the folic acid pathway. In this study, the 5‐MTHF synthetic pathway in Bacillus subtilis was systematically engineered to realize 5‐MTHF accumulation and further improve 5‐MTHF production. Specifically, the 5‐MTHF synthesis pathway with dihydrofolate (DHF) as the precursor was strengthened to shift the metabolic flux to 5‐MTHF biosynthesis by replacing the native yitJ gene with Escherichia coli metF , knockout of purU , and overexpressing dfrA . The intracellular level of 5‐MTHF increased 26.4‐fold, reaching 271.64 µg/L. Next, the 5‐MTHF precursor supply pathway was strengthened by co‐overexpression of folC, pabB, folE , and yciA . This resulted in a 93.2‐fold improvement of the 5‐MTHF titer, which reached 960.27 µg/L. Finally, the clustered regularly interspaced short palindromic repeats interference system was used to identify key genes in the competitive and catabolic pathways for repression to further shift the metabolic flux toward 5‐MTHF biosynthesis. The repression of genes thyA (existing in the purine metabolic pathway), pheA (existing in the competitive metabolic pathway), trpE (existing in the competitive metabolic pathway), and panB (existing in the pantoate synthesis pathway) significantly increased the titer of 5‐MTHF. By repressing the pheA gene, the 5‐MTHF titer reached 1.58 mg/L, which was 153.8‐fold that of the wild‐type strain of B. subtilis 168. Through medium optimization, the 5‐MTHF titer reached 1.78 mg/L, which was currently the highest titer of 5‐MTHF in B. subtilis . Apart from the highest titer of 5‐MTHF, the highest titer of total folates including 5‐MTHF, 5‐FTHF, folic acid, and THF could reach 3.31 mg/L, which was 8.5‐fold that in B. subtilis . To the best of our knowledge, the 5‐MTHF and total folate titers reported here are the highest using a Generally regarded as safe (GRAS) bacterium as the production host. Overall, this study provides a good starting point for further metabolic engineering to achieve efficient biosynthesis of 5‐MTHF by GRAS bacteria.
中文翻译:

枯草芽孢杆菌系统代谢工程用于 5-甲基四氢叶酸的有效生物合成。
5-甲基四氢叶酸 (5-MTHF) 是人体血浆中叶酸的主要形式,也是唯一可以穿透血脑屏障的叶酸形式。已被广泛用于各种疾病的预防和治疗。它主要由化学合成生产。然而,低生产率已不能满足日益增长的需求。此外,化学合成可能对环境有害。尽管有多种微生物合成 5-MTHF,但由于 5-MTHF 途径的严格调控和向叶酸途径的代谢流量有限,因此缺乏有效的 5-MTHF 生物生产方法。在本研究中,枯草芽孢杆菌中的 5-MTHF 合成途径系统地设计以实现 5-MTHF 积累并进一步提高 5-MTHF 产量。具体来说,以二氢叶酸(DHF)为前体的 5-MTHF 合成途径得到加强,通过用大肠杆菌metF替换天然yitJ基因、敲除purU和过表达dfrA将代谢通量转移到 5-MTHF 生物合成。5-MTHF 的细胞内水平增加了 26.4 倍,达到 271.64 µg/L。接下来,通过folC、pabB、folE和yciA 的共过表达加强了 5-MTHF 前体供应途径. 这导致 5-MTHF 滴度提高了 93.2 倍,达到 960.27 µg/L。最后,成簇的规则间隔短回文重复干扰系统用于识别竞争和分解代谢途径中的关键基因,以进一步将代谢通量转向 5-MTHF 生物合成。对thyA(存在于嘌呤代谢途径)、pheA(存在于竞争性代谢途径)、trpE(存在于竞争性代谢途径)和panB(存在于泛解酸合成途径中)基因的抑制显着增加了5 -MTHF。通过抑制pheA5-MTHF 滴度达到 1.58 mg/L,是野生型枯草芽孢杆菌168菌株的 153.8 倍。通过培养基优化,5-MTHF 滴度达到 1.78 mg/L,是目前枯草芽孢杆菌中 5-MTHF 的最高滴度。除了5-MTHF的最高滴度外,5-MTHF、5-FTHF、叶酸和THF等总叶酸的最高滴度可达3.31mg/L,是枯草芽孢杆菌的8.5倍。据我们所知,此处报告的 5-MTHF 和总叶酸滴度是使用公认安全 (GRAS) 细菌作为生产宿主时最高的。总的来说,这项研究为进一步的代谢工程提供了一个良好的起点,以实现 GRAS 细菌对 5-MTHF 的有效生物合成。
更新日期:2020-03-14
中文翻译:

枯草芽孢杆菌系统代谢工程用于 5-甲基四氢叶酸的有效生物合成。
5-甲基四氢叶酸 (5-MTHF) 是人体血浆中叶酸的主要形式,也是唯一可以穿透血脑屏障的叶酸形式。已被广泛用于各种疾病的预防和治疗。它主要由化学合成生产。然而,低生产率已不能满足日益增长的需求。此外,化学合成可能对环境有害。尽管有多种微生物合成 5-MTHF,但由于 5-MTHF 途径的严格调控和向叶酸途径的代谢流量有限,因此缺乏有效的 5-MTHF 生物生产方法。在本研究中,枯草芽孢杆菌中的 5-MTHF 合成途径系统地设计以实现 5-MTHF 积累并进一步提高 5-MTHF 产量。具体来说,以二氢叶酸(DHF)为前体的 5-MTHF 合成途径得到加强,通过用大肠杆菌metF替换天然yitJ基因、敲除purU和过表达dfrA将代谢通量转移到 5-MTHF 生物合成。5-MTHF 的细胞内水平增加了 26.4 倍,达到 271.64 µg/L。接下来,通过folC、pabB、folE和yciA 的共过表达加强了 5-MTHF 前体供应途径. 这导致 5-MTHF 滴度提高了 93.2 倍,达到 960.27 µg/L。最后,成簇的规则间隔短回文重复干扰系统用于识别竞争和分解代谢途径中的关键基因,以进一步将代谢通量转向 5-MTHF 生物合成。对thyA(存在于嘌呤代谢途径)、pheA(存在于竞争性代谢途径)、trpE(存在于竞争性代谢途径)和panB(存在于泛解酸合成途径中)基因的抑制显着增加了5 -MTHF。通过抑制pheA5-MTHF 滴度达到 1.58 mg/L,是野生型枯草芽孢杆菌168菌株的 153.8 倍。通过培养基优化,5-MTHF 滴度达到 1.78 mg/L,是目前枯草芽孢杆菌中 5-MTHF 的最高滴度。除了5-MTHF的最高滴度外,5-MTHF、5-FTHF、叶酸和THF等总叶酸的最高滴度可达3.31mg/L,是枯草芽孢杆菌的8.5倍。据我们所知,此处报告的 5-MTHF 和总叶酸滴度是使用公认安全 (GRAS) 细菌作为生产宿主时最高的。总的来说,这项研究为进一步的代谢工程提供了一个良好的起点,以实现 GRAS 细菌对 5-MTHF 的有效生物合成。


























 京公网安备 11010802027423号
京公网安备 11010802027423号