当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Investigation of the cathodic interfacial stability of a nitrile electrolyte and its performance with a high-voltage LiCoO2 cathode.
Chemical Communications ( IF 4.9 ) Pub Date : 2020-05-07 , DOI: 10.1039/d0cc00049c Fang Xian 1 , Jiedong Li 2 , Zhenglin Hu 2 , Qian Zhou 2 , Chen Wang 2 , Chenglong Lu 2 , Zhongyi Zhang 1 , Shanmu Dong 2 , Chunbo Mou 1 , Guanglei Cui 2
Chemical Communications ( IF 4.9 ) Pub Date : 2020-05-07 , DOI: 10.1039/d0cc00049c Fang Xian 1 , Jiedong Li 2 , Zhenglin Hu 2 , Qian Zhou 2 , Chen Wang 2 , Chenglong Lu 2 , Zhongyi Zhang 1 , Shanmu Dong 2 , Chunbo Mou 1 , Guanglei Cui 2
Affiliation
|
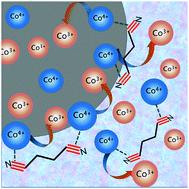
The limited discharge capacity of LiCoO2 can be improved by increasing its working potential, but it suffers from Co4+ dissolution and decomposition of the electrolyte. Nitriles have attracted great interest as high-voltage electrolytes due to their wide electrochemical window. However, the cathodic interfacial stability of nitrile electrolytes with a high-voltage LiCoO2 cathode has yet to be explored. Herein, we adopted an SN-based deep eutectic electrolyte with SN as the only solvent and found that Co4+ could be reduced by the SN solvent on the interface of the LiCoO2 electrode, causing a reverse phase change of LiCoO2 and severe self-discharge of the LiCoO2|Li and LiCoO2|Li4Ti5O12 batteries. When LiDFOB was introduced into the electrolyte, the self-discharge behavior of cells could be largely decelerated. The series of characterizations performed in our work revealed that the cathode/electrolyte interface generated from the LiDFOB salt could stabilize the interface of LiCoO2 and suppress the dissolution of the ions of the transition metal Co.
中文翻译:

丁腈电解质的阴极界面稳定性及其在高压LiCoO2阴极上的性能研究。
可以通过增加LiCoO2的工作电势来提高其有限的放电容量,但会遭受Co4 +溶解和电解质分解的困扰。丁腈由于其宽的电化学窗口而引起了人们对于高压电解液的巨大兴趣。然而,具有高压LiCoO 2阴极的腈电解质的阴极界面稳定性尚待探索。在本文中,我们采用了以SN为唯一溶剂的SN基深共熔电解质,发现SN溶剂可在LiCoO2电极界面上还原Co4 +,从而引起LiCoO2的反相变化和电极严重的自放电。 LiCoO2 | Li和LiCoO2 | Li4Ti5O12电池。当LiDFOB被引入到电解质中时,电池的自放电行为会大大降低。
更新日期:2020-03-15
中文翻译:

丁腈电解质的阴极界面稳定性及其在高压LiCoO2阴极上的性能研究。
可以通过增加LiCoO2的工作电势来提高其有限的放电容量,但会遭受Co4 +溶解和电解质分解的困扰。丁腈由于其宽的电化学窗口而引起了人们对于高压电解液的巨大兴趣。然而,具有高压LiCoO 2阴极的腈电解质的阴极界面稳定性尚待探索。在本文中,我们采用了以SN为唯一溶剂的SN基深共熔电解质,发现SN溶剂可在LiCoO2电极界面上还原Co4 +,从而引起LiCoO2的反相变化和电极严重的自放电。 LiCoO2 | Li和LiCoO2 | Li4Ti5O12电池。当LiDFOB被引入到电解质中时,电池的自放电行为会大大降低。


























 京公网安备 11010802027423号
京公网安备 11010802027423号