PLoS Pathogens ( IF 6.7 ) Pub Date : 2020-03-13 , DOI: 10.1371/journal.ppat.1008374 M Indriati Hood-Pishchany 1 , Ly Pham 2, 3 , Christiaan D Wijers 2, 3 , William J Burns 2 , Kelli L Boyd 2, 3 , Lauren D Palmer 2, 3 , Eric P Skaar 2, 3, 4 , Michael J Noto 2, 3, 5
|
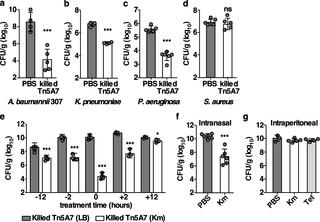
Antimicrobial resistance is increasing in pathogenic bacteria. Yet, the effect of antibiotic exposure on resistant bacteria has been underexplored and may affect pathogenesis. Here we describe the discovery that propagation of the human pathogen Acinetobacter baumannii in an aminoglycoside antibiotic results in alterations to the bacterium that interact with lung innate immunity resulting in enhanced bacterial clearance. Co-inoculation of mice with A. baumannii grown in the presence and absence of the aminoglycoside, kanamycin, induces enhanced clearance of a non-kanamycin-propagated strain. This finding can be replicated when kanamycin-propagated A. baumannii is killed prior to co-inoculation of mice, indicating the enhanced bacterial clearance results from interactions with innate host defenses in the lung. Infection with kanamycin-propagated A. baumannii alters the kinetics of phagocyte recruitment to the lung and reduces pro- and anti-inflammatory cytokine and chemokine production in the lung and blood. This culminates in reduced histopathologic evidence of lung injury during infection despite enhanced bacterial clearance. Further, the antibacterial response induced by killed aminoglycoside-propagated A. baumannii enhances the clearance of multiple clinically relevant Gram-negative pathogens from the lungs of infected mice. Together, these findings exemplify cooperation between antibiotics and the host immune system that affords protection against multiple antibiotic-resistant bacterial pathogens. Further, these findings highlight the potential for the development of a broad-spectrum therapeutic that exploits a similar mechanism to that described here and acts as an innate immunity modulator.
中文翻译:

氨基糖苷类增殖的鲍曼不动杆菌对细菌性肺炎的广谱抑制。
病原菌的抗菌素耐药性正在增加。然而,抗生素暴露对耐药菌的影响尚未得到充分探索,并可能影响发病机制。在这里,我们描述了人类病原体鲍曼不动杆菌在氨基糖苷类抗生素中的繁殖导致与肺先天免疫相互作用的细菌的改变,从而提高了细菌清除率。小鼠与A共接种。在存在和不存在氨基糖苷类卡那霉素的情况下生长的鲍曼不动杆菌诱导增强的非卡那霉素繁殖菌株的清除。当卡那霉素传播A时,可以复制这一发现。鲍曼尼在共同接种小鼠之前被杀死,表明增强的细菌清除是由于与肺中先天宿主防御的相互作用造成的。卡那霉素传播的 A感染。鲍曼不动杆菌改变吞噬细胞向肺募集的动力学,并减少肺和血液中促炎和抗炎细胞因子和趋化因子的产生。尽管细菌清除率增强,但最终导致感染期间肺损伤的组织病理学证据减少。此外,杀死氨基糖苷类增殖的 A诱导的抗菌反应。鲍曼尼增强对受感染小鼠肺部多种临床相关革兰氏阴性病原体的清除。总之,这些发现证明了抗生素与宿主免疫系统之间的合作,从而提供针对多种抗生素耐药性细菌病原体的保护。此外,这些发现突出了开发一种广谱治疗剂的潜力,该治疗剂利用与此处描述的机制相似的机制并充当先天免疫调节剂。



























 京公网安备 11010802027423号
京公网安备 11010802027423号