The Lancet Oncology ( IF 51.1 ) Pub Date : 2020-03-12 , DOI: 10.1016/s1470-2045(20)30013-9 Ursula Nestle 1 , Tanja Schimek-Jasch 2 , Stephanie Kremp 3 , Andrea Schaefer-Schuler 4 , Michael Mix 5 , Andreas Küsters 6 , Marco Tosch 7 , Thomas Hehr 8 , Susanne Martina Eschmann 9 , Yves-Pierre Bultel 10 , Peter Hass 11 , Jochen Fleckenstein 3 , Alexander Thieme 12 , Marcus Stockinger 13 , Karin Dieckmann 14 , Matthias Miederer 15 , Gabriele Holl 16 , H Christian Rischke 17 , Eleni Gkika 2 , Sonja Adebahr 18 , Jochem König 19 , Anca-Ligia Grosu 18 ,
|
Background
With increasingly precise radiotherapy and advanced medical imaging, the concept of radiotherapy target volume planning might be redefined with the aim of improving outcomes. We aimed to investigate whether target volume reduction is feasible and effective compared with conventional planning in the context of radical chemoradiotherapy for patients with locally advanced non-small-cell lung cancer.
Methods
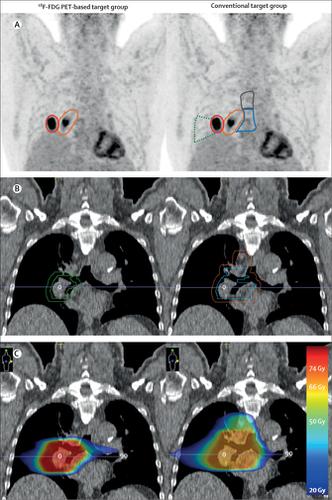
We did a multicentre, open-label, randomised, controlled trial (PET-Plan; ARO-2009-09) in 24 centres in Austria, Germany, and Switzerland. Previously untreated patients (aged older than 18 years) with inoperable locally advanced non-small-cell lung cancer suitable for chemoradiotherapy and an Eastern Cooperative Oncology Group performance status of less than 3 were included. Undergoing 18F-fluorodeoxyglucose (18F-FDG) PET and CT for treatment planning, patients were randomly assigned (1:1) using a random number generator and block sizes between four and six to target volume delineation informed by 18F-FDG PET and CT plus elective nodal irradiation (conventional target group) or target volumes informed by PET alone (18F-FDG PET-based target group). Randomisation was stratified by centre and Union for International Cancer Control stage. In both groups, dose-escalated radiotherapy (60–74 Gy, 2 Gy per fraction) was planned to the respective target volumes and applied with concurrent platinum-based chemotherapy. The primary endpoint was time to locoregional progression from randomisation with the objective to test non-inferiority of 18F-FDG PET-based planning with a prespecified hazard ratio (HR) margin of 1·25. The per-protocol set was included in the primary analysis. The safety set included all patients receiving any study-specific treatment. Patients and study staff were not masked to treatment assignment. This study is registered with ClinicalTrials.gov, NCT00697333.
Findings
From May 13, 2009, to Dec 5, 2016, 205 of 311 recruited patients were randomly assigned to the conventional target group (n=99) or the 18F-FDG PET-based target group (n=106; the intention-to-treat set), and 172 patients were treated per protocol (84 patients in the conventional target group and 88 in the 18F-FDG PET-based target group). At a median follow-up of 29 months (IQR 9–54), the risk of locoregional progression in the 18F-FDG PET-based target group was non-inferior to, and in fact lower than, that in the conventional target group in the per-protocol set (14% [95% CI 5–21] vs 29% [17–38] at 1 year; HR 0·57 [95% CI 0·30–1·06]). The risk of locoregional progression in the 18F-FDG PET-based target group was also non-inferior to that in the conventional target group in the intention-to-treat set (17% [95% CI 9–24] vs 30% [20–39] at 1 year; HR 0·64 [95% CI 0·37–1·10]). The most common acute grade 3 or worse toxicity was oesophagitis or dysphagia (16 [16%] of 99 patients in the conventional target group vs 17 [16%] of 105 patients in the 18F-FDG PET-based target group); the most common late toxicities were lung-related (12 [12%] vs 11 [10%]). 20 deaths potentially related to study treatment were reported (seven vs 13).
Interpretation
18F-FDG PET-based planning could potentially improve local control and does not seem to increase toxicity in patients with chemoradiotherapy-treated locally advanced non-small-cell lung cancer. Imaging-based target volume reduction in this setting is, therefore, feasible, and could potentially be considered standard of care. The procedures established might also support imaging-based target volume reduction concepts for other tumours.
Funding
German Cancer Aid (Deutsche Krebshilfe).
中文翻译:

局部晚期非小细胞肺癌(PET-Plan)放化疗中基于影像学的靶标体积减少:一项多中心,开放标签,随机对照试验。
背景
随着放射治疗的日益精确和先进的医学成像,可能需要重新定义放射治疗目标体积计划的概念,以改善疗效。我们旨在研究局部放疗对局部晚期非小细胞肺癌患者而言,与常规计划相比,减少靶区体积是否可行和有效。
方法
我们在奥地利,德国和瑞士的24个中心进行了一项多中心,开放标签,随机对照试验(PET-Plan; ARO-2009-09)。包括先前未接受过治疗且无法手术的局部晚期非小细胞肺癌的患者(年龄大于18岁),这些患者适合放化疗,并且东部合作肿瘤小组的表现状态低于3。进行18 F-氟脱氧葡萄糖(18 F-FDG)PET和CT的治疗计划后,使用随机数发生器将患者随机分配(1:1),块大小在4到6之间,以18 F-FDG PET告知目标体积和CT加上选择性淋巴结照射(常规目标人群)或仅由PET告知的目标体积(18基于F-FDG PET的目标群体)。中心和国际癌症控制联合会对随机分组进行了分层。在两组中,均已计划将剂量递增放疗(60-74 Gy,每部分2 Gy)规划为各自的目标体积,并同时进行铂类化学疗法。主要终点是从随机区域发展到局部区域的时间,目的是检验基于18 F-FDG PET的计划的非劣效性,预先确定的危险比(HR)裕度为1·25。每个协议集都包含在主要分析中。安全设置包括所有接受任何针对研究的治疗的患者。患者和研究人员并未隐瞒治疗任务。该研究已在ClinicalTrials.gov注册,NCT00697333。
发现
从2009年5月13日到2016年12月5日,在311名入选患者中,有205名被随机分配到常规目标组(n = 99)或18个基于F-FDG PET的目标组(n = 106;治疗组),按方案治疗172例患者(常规靶组84例,18 F-FDG PET靶组88例)。在中位随访29个月(IQR 9-54)时,基于18 F-FDG PET的目标人群局部区域进展的风险不低于传统目标人群,但实际上低于传统目标人群在每个协议集(14%[95%CI 5-21] vs 29%[17-38]在1年时; HR 0·57 [95%CI 0·30-1·06])。18岁局部区域进展的风险基于F-FDG PET的目标人群在意向治疗组中也不逊于常规目标人群(1年时17%[95%CI 9-24] vs 30%[20-39]) ; HR 0·64 [95%CI 0·37-1·10])。最常见的急性3级或更糟糕的毒性为食管炎或(99个例的常规目标组中16 [16%]吞咽困难VS 105例中17 [16%] 18基于PET-F-FDG目标基); 最常见的晚期毒性是与肺有关的(12 [12%]比11 [10%])。报告了20例可能与研究治疗相关的死亡(七对13)。
解释
18基于F-FDG PET的计划可能会改善局部控制,而且似乎不会增加接受放化疗治疗的局部晚期非小细胞肺癌患者的毒性。因此,在这种情况下基于影像的目标体积减少是可行的,并有可能被视为护理标准。建立的程序可能还支持针对其他肿瘤的基于成像的目标体积减少概念。
资金
德国癌症援助组织(Deutsche Krebshilfe)。



























 京公网安备 11010802027423号
京公网安备 11010802027423号