Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Rifampicin and clarithromycin (extended release) versus rifampicin and streptomycin for limited Buruli ulcer lesions: a randomised, open-label, non-inferiority phase 3 trial.
The Lancet ( IF 168.9 ) Pub Date : 2020-03-12 , DOI: 10.1016/s0140-6736(20)30047-7 Richard O Phillips 1 , Jérôme Robert 2 , Kabiru Mohamed Abass 3 , William Thompson 3 , Fred Stephen Sarfo 1 , Tuah Wilson 4 , Godfred Sarpong 5 , Thierry Gateau 6 , Annick Chauty 6 , Raymond Omollo 7 , Michael Ochieng Otieno 7 , Thaddaeus W Egondi 7 , Edwin O Ampadu 8 , Didier Agossadou 9 , Estelle Marion 10 , Line Ganlonon 6 , Mark Wansbrough-Jones 11 , Jacques Grosset 12 , John M Macdonald 13 , Terry Treadwell 14 , Paul Saunderson 15 , Albert Paintsil 16 , Linda Lehman 15 , Michael Frimpong 1 , Nanaa Francisca Sarpong 1 , Raoul Saizonou 17 , Alexandre Tiendrebeogo 18 , Sally-Ann Ohene 19 , Ymkje Stienstra 20 , Kingsley B Asiedu 21 , Tjip S van der Werf 20 ,
The Lancet ( IF 168.9 ) Pub Date : 2020-03-12 , DOI: 10.1016/s0140-6736(20)30047-7 Richard O Phillips 1 , Jérôme Robert 2 , Kabiru Mohamed Abass 3 , William Thompson 3 , Fred Stephen Sarfo 1 , Tuah Wilson 4 , Godfred Sarpong 5 , Thierry Gateau 6 , Annick Chauty 6 , Raymond Omollo 7 , Michael Ochieng Otieno 7 , Thaddaeus W Egondi 7 , Edwin O Ampadu 8 , Didier Agossadou 9 , Estelle Marion 10 , Line Ganlonon 6 , Mark Wansbrough-Jones 11 , Jacques Grosset 12 , John M Macdonald 13 , Terry Treadwell 14 , Paul Saunderson 15 , Albert Paintsil 16 , Linda Lehman 15 , Michael Frimpong 1 , Nanaa Francisca Sarpong 1 , Raoul Saizonou 17 , Alexandre Tiendrebeogo 18 , Sally-Ann Ohene 19 , Ymkje Stienstra 20 , Kingsley B Asiedu 21 , Tjip S van der Werf 20 ,
Affiliation
|
BACKGROUND
Buruli ulcer is a neglected tropical disease caused by Mycobacterium ulcerans infection that damages the skin and subcutis. It is most prevalent in western and central Africa and Australia. Standard antimicrobial treatment with oral rifampicin 10 mg/kg plus intramuscular streptomycin 15 mg/kg once daily for 8 weeks (RS8) is highly effective, but streptomycin injections are painful and potentially harmful. We aimed to compare the efficacy and tolerability of fully oral rifampicin 10 mg/kg plus clarithromycin 15 mg/kg extended release once daily for 8 weeks (RC8) with that of RS8 for treatment of early Buruli ulcer lesions.
METHODS
We did an open-label, non-inferiority, randomised (1:1 with blocks of six), multicentre, phase 3 clinical trial comparing fully oral RC8 with RS8 in patients with early, limited Buruli ulcer lesions. There were four trial sites in hospitals in Ghana (Agogo, Tepa, Nkawie, Dunkwa) and one in Benin (Pobè). Participants were included if they were aged 5 years or older and had typical Buruli ulcer with no more than one lesion (caterories I and II) no larger than 10 cm in diameter. The trial was open label, and neither the investigators who took measurements of the lesions nor the attending doctors were masked to treatment assignment. The primary clinical endpoint was lesion healing (ie, full epithelialisation or stable scar) without recurrence at 52 weeks after start of antimicrobial therapy. The primary endpoint and safety were assessed in the intention-to-treat population. A sample size of 332 participants was calculated to detect inferiority of RC8 by a margin of 12%. This study was registered with ClinicalTrials.gov, NCT01659437.
FINDINGS
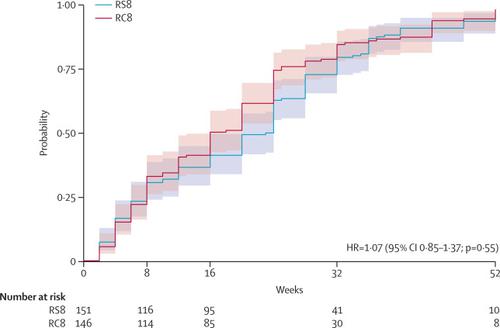
Between Jan 1, 2013, and Dec 31, 2017, participants were recruited to the trial. We stopped recruitment after 310 participants. Median age of participants was 14 years (IQR 10-29) and 153 (52%) were female. 297 patients had PCR-confirmed Buruli ulcer; 151 (51%) were assigned to RS8 treatment, and 146 (49%) received oral RC8 treatment. In the RS8 group, lesions healed in 144 (95%, 95% CI 91 to 98) of 151 patients, whereas lesions healed in 140 (96%, 91 to 99) of 146 patients in the RC8 group. The difference in proportion, -0·5% (-5·2 to 4·2), was not significantly greater than zero (p=0·59), showing that RC8 treatment is non-inferior to RS8 treatment for lesion healing at 52 weeks. Treatment-related adverse events were recorded in 20 (13%) patients receiving RS8 and in nine (7%) patients receiving RC8. Most adverse events were grade 1-2, but one (1%) patient receiving RS8 developed serious ototoxicity and ended treatment after 6 weeks. No patients needed surgical resection. Four patients (two in each study group) had skin grafts.
INTERPRETATION
Fully oral RC8 regimen was non-inferior to RS8 for treatment of early, limited Buruli ulcer and was associated with fewer adverse events. Therefore, we propose that fully oral RC8 should be the preferred therapy for early, limited lesions of Buruli ulcer.
FUNDING
WHO with additional support from MAP International, American Leprosy Missions, Fondation Raoul Follereau France, Buruli ulcer Groningen Foundation, Sanofi-Pasteur, and BuruliVac.
中文翻译:

利福平和克拉霉素(延长释放)与利福平和链霉素在有限的布鲁氏溃疡病变中的作用:一项随机,开放标签,非自卑性的3期临床试验。
背景技术布鲁氏溃疡是由溃疡分枝杆菌感染引起的被忽视的热带疾病,其损害皮肤和皮下组织。它在非洲中西部和澳大利亚最为普遍。口服利福平10 mg / kg加肌内链霉素15 mg / kg每天一次,持续8周(RS8),是标准的抗菌治疗,非常有效,但链霉素注射会带来痛苦,并且可能有害。我们旨在比较10 mg / kg的利福平和15 mg / kg的克拉霉素的全口服缓释药与RS8相比,每天一次,持续8周(RC8)的疗效和耐受性,以治疗早期Buruli溃疡病灶。方法我们进行了一项开放性,非自卑性,随机(1:1,六个区块),多中心,3期临床试验,比较了早期局限性布鲁氏溃疡病患者的完全口服RC8和RS8。加纳的医院(阿戈戈,特帕,恩卡维,敦克瓦)有四个试验点,贝宁的医院有1个试验点(波贝)。如果参与者年龄大于或等于5岁且患有典型的Buruli溃疡,且病变直径不超过10厘米(I类和II类),则包括参与者。该试验是公开的,既没有对病灶进行测量的研究人员,也没有对主治医生进行掩护。主要的临床终点是病灶愈合(即完全上皮化或稳定的瘢痕),开始抗菌治疗后52周未复发。在意向性治疗人群中评估了主要终点和安全性。计算了332名参与者的样本量,以发现RC8的劣质性为12%。该研究已在ClinicalTrials.gov注册,NCT01659437。结果在2013年1月1日至2017年12月31日之间,我们招募了参与者。310名参与者后,我们停止了招聘。参与者的中位年龄为14岁(IQR 10-29),女性为153名(52%)。297例经PCR证实的布鲁氏溃疡;151名患者(51%)被指定接受RS8治疗,而146名患者(49%)被接受口服RC8治疗。在RS8组中,151例患者中的144例(95%,95%CI 91至98)治愈,而在RC8组中146例患者中,140例(96%,91至99%)治愈。比例差异-0·5%(-5·2至4·2)并未显着大于零(p = 0·59),表明RC8治疗在90℃时的病灶愈合不逊于RS8治疗。 52周。在20例(13%)接受RS8的患者和9例(7%)接受RC8的患者中记录了与治疗相关的不良事件。大多数不良事件为1-2级,但接受RS8的一名(1%)患者出现严重的耳毒性并在6周后终止治疗。没有患者需要手术切除。四名患者(每个研究组两名)进行了植皮。解释完全口服RC8方案在治疗早期局限性Buruli溃疡方面不逊于RS8,并且不良事件较少。因此,我们建议完全口服RC8应该是Buruli溃疡早期,局限性病变的首选治疗方法。在MAP国际,美国麻风病基金会,法国拉乌尔·福勒罗基金会,布鲁里溃疡格罗宁根基金会,赛诺菲-巴斯德和布鲁里瓦克的额外支持下,向世卫组织提供资金。解释完全的口服RC8方案在治疗早期局限性Buruli溃疡方面不逊于RS8,并且不良事件较少。因此,我们建议完全口服RC8应该是布鲁氏溃疡早期,局限性病变的首选治疗方法。在MAP国际,美国麻风病基金会,法国拉乌尔·福勒罗基金会,布鲁里溃疡格罗宁根基金会,赛诺菲-巴斯德和布鲁里瓦克的额外支持下,向世卫组织提供资金。解释完全口服RC8方案在治疗早期局限性Buruli溃疡方面不逊于RS8,并且不良事件较少。因此,我们建议完全口服RC8应该是Buruli溃疡早期,局限性病变的首选治疗方法。在MAP国际,美国麻风病基金会,法国拉乌尔·福勒罗基金会,布鲁里溃疡格罗宁根基金会,赛诺菲-巴斯德和布鲁里瓦克的额外支持下,向世卫组织提供资金。
更新日期:2020-04-22
中文翻译:

利福平和克拉霉素(延长释放)与利福平和链霉素在有限的布鲁氏溃疡病变中的作用:一项随机,开放标签,非自卑性的3期临床试验。
背景技术布鲁氏溃疡是由溃疡分枝杆菌感染引起的被忽视的热带疾病,其损害皮肤和皮下组织。它在非洲中西部和澳大利亚最为普遍。口服利福平10 mg / kg加肌内链霉素15 mg / kg每天一次,持续8周(RS8),是标准的抗菌治疗,非常有效,但链霉素注射会带来痛苦,并且可能有害。我们旨在比较10 mg / kg的利福平和15 mg / kg的克拉霉素的全口服缓释药与RS8相比,每天一次,持续8周(RC8)的疗效和耐受性,以治疗早期Buruli溃疡病灶。方法我们进行了一项开放性,非自卑性,随机(1:1,六个区块),多中心,3期临床试验,比较了早期局限性布鲁氏溃疡病患者的完全口服RC8和RS8。加纳的医院(阿戈戈,特帕,恩卡维,敦克瓦)有四个试验点,贝宁的医院有1个试验点(波贝)。如果参与者年龄大于或等于5岁且患有典型的Buruli溃疡,且病变直径不超过10厘米(I类和II类),则包括参与者。该试验是公开的,既没有对病灶进行测量的研究人员,也没有对主治医生进行掩护。主要的临床终点是病灶愈合(即完全上皮化或稳定的瘢痕),开始抗菌治疗后52周未复发。在意向性治疗人群中评估了主要终点和安全性。计算了332名参与者的样本量,以发现RC8的劣质性为12%。该研究已在ClinicalTrials.gov注册,NCT01659437。结果在2013年1月1日至2017年12月31日之间,我们招募了参与者。310名参与者后,我们停止了招聘。参与者的中位年龄为14岁(IQR 10-29),女性为153名(52%)。297例经PCR证实的布鲁氏溃疡;151名患者(51%)被指定接受RS8治疗,而146名患者(49%)被接受口服RC8治疗。在RS8组中,151例患者中的144例(95%,95%CI 91至98)治愈,而在RC8组中146例患者中,140例(96%,91至99%)治愈。比例差异-0·5%(-5·2至4·2)并未显着大于零(p = 0·59),表明RC8治疗在90℃时的病灶愈合不逊于RS8治疗。 52周。在20例(13%)接受RS8的患者和9例(7%)接受RC8的患者中记录了与治疗相关的不良事件。大多数不良事件为1-2级,但接受RS8的一名(1%)患者出现严重的耳毒性并在6周后终止治疗。没有患者需要手术切除。四名患者(每个研究组两名)进行了植皮。解释完全口服RC8方案在治疗早期局限性Buruli溃疡方面不逊于RS8,并且不良事件较少。因此,我们建议完全口服RC8应该是Buruli溃疡早期,局限性病变的首选治疗方法。在MAP国际,美国麻风病基金会,法国拉乌尔·福勒罗基金会,布鲁里溃疡格罗宁根基金会,赛诺菲-巴斯德和布鲁里瓦克的额外支持下,向世卫组织提供资金。解释完全的口服RC8方案在治疗早期局限性Buruli溃疡方面不逊于RS8,并且不良事件较少。因此,我们建议完全口服RC8应该是布鲁氏溃疡早期,局限性病变的首选治疗方法。在MAP国际,美国麻风病基金会,法国拉乌尔·福勒罗基金会,布鲁里溃疡格罗宁根基金会,赛诺菲-巴斯德和布鲁里瓦克的额外支持下,向世卫组织提供资金。解释完全口服RC8方案在治疗早期局限性Buruli溃疡方面不逊于RS8,并且不良事件较少。因此,我们建议完全口服RC8应该是Buruli溃疡早期,局限性病变的首选治疗方法。在MAP国际,美国麻风病基金会,法国拉乌尔·福勒罗基金会,布鲁里溃疡格罗宁根基金会,赛诺菲-巴斯德和布鲁里瓦克的额外支持下,向世卫组织提供资金。



























 京公网安备 11010802027423号
京公网安备 11010802027423号