当前位置:
X-MOL 学术
›
Chem. Geol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Chemodenitrification by Fe(II) and nitrite: pH effect, mineralization and kinetic modeling
Chemical Geology ( IF 3.9 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.chemgeo.2020.119586 Dandan Chen , Xiu Yuan , Wenqi Zhao , Xiaobo Luo , Fangbai Li , Tongxu Liu
Chemical Geology ( IF 3.9 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.chemgeo.2020.119586 Dandan Chen , Xiu Yuan , Wenqi Zhao , Xiaobo Luo , Fangbai Li , Tongxu Liu
|
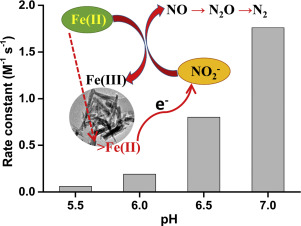
Abstract Despite the biogeochemical significance of redox interactions between nitrite (NO2−) and Fe(II) species, considerable uncertainty still remains as to the exact processes contributing to the rates and extents of such chemodenitrification processes and its associated importance to the global N2O flux. In this study, pH effect and mineral formation on the redox interactions between NO2− and Fe(II) under anaerobic conditions over the pH range 5.5–7.0 were investigated, with a detailed kinetic model developed to describe the predominant mechanisms operating in the system. Our results demonstrated that chemodenitrification proceeds without an initial solid phase and both Fe(II) oxidation and NO2− reduction rates increased with pH increasing from 5.5 to 7.0, with concomitant generation of N2O. While the oxidation of Fe(II) leads to a rapid formation of goethite and shifts the reaction into a heterogeneous mechanism, N2O detected in this study only partially account for the NO2− consumed throughout the entire examined pH range. The developed model indicated that both the dissolved and surface-associated Fe(II) species were kinetically active toward nitrite reduction, indicating the presence of Fe(III) minerals acting as a positive feedback and auto-catalytic pathway for the reaction. The kinetic model may provide critical insight into the underlying mechanisms and relative contribution of chemodenitrification to biological nitrite reduction and assist in understanding and prediction on the chemical aspects controlling iron and nitrite transformation in environments exhibiting rapidly fluctuating redox conditions.
中文翻译:

Fe(II) 和亚硝酸盐的化学硝化作用:pH 值效应、矿化和动力学模型
摘要 尽管亚硝酸盐 (NO2−) 和 Fe(II) 物种之间的氧化还原相互作用具有生物地球化学意义,但对于促成此类化学脱氮过程的速率和程度的确切过程及其对全球 N2O 通量的相关重要性,仍然存在相当大的不确定性。在这项研究中,研究了 pH 值范围 5.5-7.0 在厌氧条件下对 NO2− 和 Fe(II) 之间氧化还原相互作用的 pH 值影响和矿物质形成,并开发了详细的动力学模型来描述系统中运行的主要机制。我们的结果表明,化学脱氮在没有初始固相的情况下进行,并且 Fe(II) 氧化和 NO2− 还原率随着 pH 从 5.5 增加到 7.0 而增加,同时产生 N2O。虽然 Fe(II) 的氧化导致针铁矿的快速形成并将反应转变为异质机制,但本研究中检测到的 N2O 仅部分解释了整个检测 pH 范围内消耗的 NO2-。开发的模型表明,溶解的和表面结合的 Fe(II) 物质对亚硝酸盐还原具有动力学活性,表明 Fe(III) 矿物的存在作为反应的正反馈和自催化途径。该动力学模型可以提供对化学脱硝化对生物亚硝酸盐还原的潜在机制和相对贡献的重要洞察,并有助于理解和预测在表现出快速波动的氧化还原条件的环境中控制铁和亚硝酸盐转化的化学方面。
更新日期:2020-05-01
中文翻译:

Fe(II) 和亚硝酸盐的化学硝化作用:pH 值效应、矿化和动力学模型
摘要 尽管亚硝酸盐 (NO2−) 和 Fe(II) 物种之间的氧化还原相互作用具有生物地球化学意义,但对于促成此类化学脱氮过程的速率和程度的确切过程及其对全球 N2O 通量的相关重要性,仍然存在相当大的不确定性。在这项研究中,研究了 pH 值范围 5.5-7.0 在厌氧条件下对 NO2− 和 Fe(II) 之间氧化还原相互作用的 pH 值影响和矿物质形成,并开发了详细的动力学模型来描述系统中运行的主要机制。我们的结果表明,化学脱氮在没有初始固相的情况下进行,并且 Fe(II) 氧化和 NO2− 还原率随着 pH 从 5.5 增加到 7.0 而增加,同时产生 N2O。虽然 Fe(II) 的氧化导致针铁矿的快速形成并将反应转变为异质机制,但本研究中检测到的 N2O 仅部分解释了整个检测 pH 范围内消耗的 NO2-。开发的模型表明,溶解的和表面结合的 Fe(II) 物质对亚硝酸盐还原具有动力学活性,表明 Fe(III) 矿物的存在作为反应的正反馈和自催化途径。该动力学模型可以提供对化学脱硝化对生物亚硝酸盐还原的潜在机制和相对贡献的重要洞察,并有助于理解和预测在表现出快速波动的氧化还原条件的环境中控制铁和亚硝酸盐转化的化学方面。



























 京公网安备 11010802027423号
京公网安备 11010802027423号