当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Discovery and Pharmacokinetics of Sulfamides and Guanidines as Potent Human Arginase 1 Inhibitors.
ACS Medicinal Chemistry Letters ( IF 4.2 ) Pub Date : 2020-03-13 , DOI: 10.1021/acsmedchemlett.9b00508 Roman Blaszczyk 1 , Joanna Brzezinska 1 , Barbara Dymek 1 , Paulina S Stanczak 1 , Marcin Mazurkiewicz 1 , Jacek Olczak 1 , Julita Nowicka 1 , Karolina Dzwonek 1 , Agnieszka Zagozdzon 1 , Jakub Golab 2 , Adam Golebiowski 1
ACS Medicinal Chemistry Letters ( IF 4.2 ) Pub Date : 2020-03-13 , DOI: 10.1021/acsmedchemlett.9b00508 Roman Blaszczyk 1 , Joanna Brzezinska 1 , Barbara Dymek 1 , Paulina S Stanczak 1 , Marcin Mazurkiewicz 1 , Jacek Olczak 1 , Julita Nowicka 1 , Karolina Dzwonek 1 , Agnieszka Zagozdzon 1 , Jakub Golab 2 , Adam Golebiowski 1
Affiliation
|
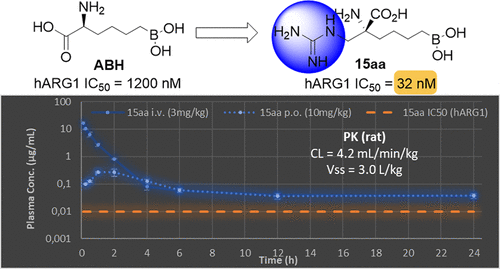
We designed and synthesized a series of arginase inhibitors as derivatives of the well-known 2-(S)-amino-6-boronohexanoic acid (ABH) with basic and neutral side chains in the α-position relative to the amino acid group. In an effort to improve the pharmacokinetic profile of literature examples and retain potent enzymatic activity, sulfamido moieties were introduced to generate hydrogen bond interaction with the aspartic acid residue in the arginase active site. The compounds with basic guanidine-containing side chains were even more potent arginase inhibitors. Both groups of compounds, as designed, demonstrated low clearance in their pharmacokinetic profile. The most active inhibitor 15aa showed high nanomolar potency with IC50 = 32 nM toward human arginase 1 and demonstrated low clearance (4.2 mL/min/kg), long t 1/2, and moderate volume of distribution in rat pharmacokinetic studies.
中文翻译:

磺胺类和胍类作为有效的人类精氨酸酶1抑制剂的发现和药代动力学。
我们设计和合成了一系列精氨酸酶抑制剂,它们是众所周知的2-(S)-氨基-6-硼己酸(ABH)的衍生物,相对于氨基酸基团在α位具有碱性和中性侧链。为了改善文献实例的药代动力学并保持有效的酶促活性,引入了磺酰胺基部分以与精氨酸酶活性位点中的天冬氨酸残基产生氢键相互作用。具有碱性胍基侧链的化合物甚至是更有效的精氨酸酶抑制剂。设计的两组化合物在药代动力学方面均显示出低清除率。活性最高的抑制剂15aa对人精氨酸酶1的纳摩尔浓度高,IC50 = 32 nM,清除率低(4.2 mL / min / kg),t 1/2长,
更新日期:2020-04-23
中文翻译:

磺胺类和胍类作为有效的人类精氨酸酶1抑制剂的发现和药代动力学。
我们设计和合成了一系列精氨酸酶抑制剂,它们是众所周知的2-(S)-氨基-6-硼己酸(ABH)的衍生物,相对于氨基酸基团在α位具有碱性和中性侧链。为了改善文献实例的药代动力学并保持有效的酶促活性,引入了磺酰胺基部分以与精氨酸酶活性位点中的天冬氨酸残基产生氢键相互作用。具有碱性胍基侧链的化合物甚至是更有效的精氨酸酶抑制剂。设计的两组化合物在药代动力学方面均显示出低清除率。活性最高的抑制剂15aa对人精氨酸酶1的纳摩尔浓度高,IC50 = 32 nM,清除率低(4.2 mL / min / kg),t 1/2长,



























 京公网安备 11010802027423号
京公网安备 11010802027423号