当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A New Docking Domain Type in the Peptide-Antimicrobial-Xenorhabdus Peptide Producing Nonribosomal Peptide Synthetase from Xenorhabdus bovienii.
ACS Chemical Biology ( IF 4 ) Pub Date : 2020-03-25 , DOI: 10.1021/acschembio.9b01022 Jonas Watzel 1 , Carolin Hacker 2 , Elke Duchardt-Ferner 2 , Helge B Bode 1, 3, 4 , Jens Wöhnert 2
ACS Chemical Biology ( IF 4 ) Pub Date : 2020-03-25 , DOI: 10.1021/acschembio.9b01022 Jonas Watzel 1 , Carolin Hacker 2 , Elke Duchardt-Ferner 2 , Helge B Bode 1, 3, 4 , Jens Wöhnert 2
Affiliation
|
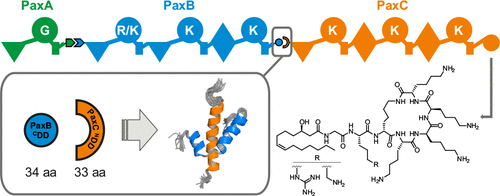
Nonribosomal peptide synthetases (NRPSs) produce a wide variety of different natural products from amino acid precursors. In contrast to single protein NRPS, the NRPS of the bacterium Xenorhabdus bovienii producing the peptide-antimicrobial-Xenorhabdus (PAX) peptide consists of three individual proteins (PaxA/B/C), which interact with each other noncovalently in a linear fashion. The specific interactions between the three different proteins in this NRPS system are mediated by short C- and N-terminal docking domains (C/NDDs). Here, we investigate the structural basis for the specific interaction between the CDD from the protein PaxB and the NDD from PaxC. The isolated DD peptides feature transient α-helical conformations in the absence of the respective DD partner. Isothermal titration calorimetry (ITC) and nuclear magnetic resonance (NMR) titration experiments showed that the two isolated DDs bind to each other and form a structurally well-defined complex with a dissociation constant in the micromolar range as is typical for many DD interactions. Artificial linking of this DD pair via a flexible glycine-serine (GS) linker enabled us to solve the structure of the DD complex by NMR spectroscopy. In the complex, the two DDs interact with each other by forming a three helix bundle arranged in an overall coiled-coil motif. Key interacting residues were identified in mutagenesis experiments. Overall, our structure of the PaxB CDD/PaxC NDD complex represents an architecturally new type of DD interaction motif.
中文翻译:

肽-抗微生物-希诺氏杆菌肽中的一种新的对接域类型,可从牛副杆菌产生非核糖体肽合成酶。
非核糖体肽合成酶(NRPS)可从氨基酸前体产生多种不同的天然产物。与单一蛋白质NRPS相比,产生肽-抗微生物-Xenorhabdus(PAX)肽的牛生线杆菌(Xenorhabdus bovienii)的NRPS由三个单独的蛋白质(PaxA / B / C)组成,它们以线性方式彼此非共价相互作用。NRPS系统中三种不同蛋白质之间的特异性相互作用是由短的C和N末端对接结构域(C / NDD)介导的。在这里,我们研究蛋白质PaxB的CDD与PaxC的NDD之间特异性相互作用的结构基础。在没有相应的DD伴侣的情况下,分离的DD肽具有瞬时α-螺旋构象。等温滴定热法(ITC)和核磁共振(NMR)滴定实验表明,两个分离的DD相互结合并形成结构明确的复合物,其解离常数在许多DD相互作用中都很典型。通过柔性甘氨酸-丝氨酸(GS)接头人工连接该DD对,使我们能够通过NMR光谱解析DD配合物的结构。在复合体中,两个DD通过形成三个螺旋束而相互作用,这些螺旋束排列成整个螺旋线圈图案。在诱变实验中确定了关键的相互作用残基。总体而言,我们PaxB CDD / PaxC NDD复合物的结构代表了一种新型的DD相互作用基序。
更新日期:2020-04-23
中文翻译:

肽-抗微生物-希诺氏杆菌肽中的一种新的对接域类型,可从牛副杆菌产生非核糖体肽合成酶。
非核糖体肽合成酶(NRPS)可从氨基酸前体产生多种不同的天然产物。与单一蛋白质NRPS相比,产生肽-抗微生物-Xenorhabdus(PAX)肽的牛生线杆菌(Xenorhabdus bovienii)的NRPS由三个单独的蛋白质(PaxA / B / C)组成,它们以线性方式彼此非共价相互作用。NRPS系统中三种不同蛋白质之间的特异性相互作用是由短的C和N末端对接结构域(C / NDD)介导的。在这里,我们研究蛋白质PaxB的CDD与PaxC的NDD之间特异性相互作用的结构基础。在没有相应的DD伴侣的情况下,分离的DD肽具有瞬时α-螺旋构象。等温滴定热法(ITC)和核磁共振(NMR)滴定实验表明,两个分离的DD相互结合并形成结构明确的复合物,其解离常数在许多DD相互作用中都很典型。通过柔性甘氨酸-丝氨酸(GS)接头人工连接该DD对,使我们能够通过NMR光谱解析DD配合物的结构。在复合体中,两个DD通过形成三个螺旋束而相互作用,这些螺旋束排列成整个螺旋线圈图案。在诱变实验中确定了关键的相互作用残基。总体而言,我们PaxB CDD / PaxC NDD复合物的结构代表了一种新型的DD相互作用基序。



























 京公网安备 11010802027423号
京公网安备 11010802027423号