当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Chelation-Induced Reversal of Negative Cation Transference Number in Ionic Liquid Electrolytes
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2020-03-19 , DOI: 10.1021/acs.jpcb.0c01089 Nicola Molinari 1 , Boris Kozinsky 1
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2020-03-19 , DOI: 10.1021/acs.jpcb.0c01089 Nicola Molinari 1 , Boris Kozinsky 1
Affiliation
|
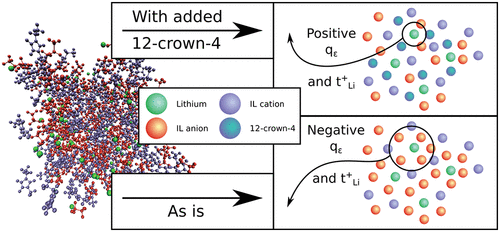
Strong anion–cation interaction in lithium-salt/ionic liquid electrolytes leads to ionic association that decreases the Li transference number, even causing it to be negative. We show that these interactions can be greatly reduced by adding cyclic ethylene oxide molecules, and we quantitatively examine the effect using rigorous multispecies concentrated solution theory coupled with molecular dynamics simulations. The added molecules, primarily lithium ionophore V also known as 12-crown-4, have high affinity to lithium, therefore disrupting the lithium cation–anion coupling, resulting in a significantly improved transference number. First, we investigate the lithium–anion spatial correlation by studying their clusters and show that the 12-crown-4 ether allows the formation of previously nonexisting positively charged lithium-containing complexes. We then prove that the chelators actively compete with the anion to coordinate lithium ions by showing that the persistence-over-time of a given anion coordination cage decreases when ionophore molecules are added to the system. Last, we report an increase in the lithium transference number for a variety of chemistries as a function of added 12-crown-4 (and another ionophore, 18-crown-6) molecules, and even positive values can be reached. Our results provide a foundation for new design and optimization strategies to reverse the sign of and increase the transference number in highly correlated concentrated electrolytes.
中文翻译:

螯合诱导的离子液体电解质中负阳离子转移数的逆转
锂盐/离子液体电解质中强烈的阴离子-阳离子相互作用会导致离子缔合,从而降低Li迁移数,甚至使其变为负数。我们表明,通过添加环状环氧乙烷分子可以大大减少这些相互作用,并且我们使用严格的多物种浓缩溶液理论和分子动力学模拟来定量研究其影响。添加的分子,主要是锂离子载体V,也称为12-crown-4,对锂具有很高的亲和力,因此破坏了锂阳离子与阴离子的偶联,从而显着提高了转移数。首先,我们通过研究它们的簇来研究锂-阴离子的空间相关性,并表明12冠4醚可以形成以前不存在的带正电的含锂配合物。然后,我们证明了当将离子载体分子添加到系统中时,给定阴离子配位笼的持续时间会减少,从而螯合剂与阴离子积极竞争来配位锂离子。最后,我们报告了随着添加的12-crown-4(和另一个离子载体,18-crown-6)分子的作用,各种化学物质的锂转移数增加,甚至可以达到正值。我们的结果为新设计和优化策略提供了基础,该策略可以逆转高度相关的浓缩电解质的符号并增加其迁移数。我们报告说,随着添加的12-crown-4(和另一个离子载体,18-crown-6)分子的作用,各种化学物质的锂转移数增加,甚至可以达到正值。我们的结果为新设计和优化策略提供了基础,该策略可以逆转高度相关的浓缩电解质的符号并增加其迁移数。我们报告说,随着添加的12-crown-4(和另一个离子载体,18-crown-6)分子的作用,各种化学物质的锂转移数增加,甚至可以达到正值。我们的结果为新设计和优化策略提供了基础,该策略可以逆转高度相关的浓缩电解质的符号并增加其迁移数。
更新日期:2020-03-20
中文翻译:

螯合诱导的离子液体电解质中负阳离子转移数的逆转
锂盐/离子液体电解质中强烈的阴离子-阳离子相互作用会导致离子缔合,从而降低Li迁移数,甚至使其变为负数。我们表明,通过添加环状环氧乙烷分子可以大大减少这些相互作用,并且我们使用严格的多物种浓缩溶液理论和分子动力学模拟来定量研究其影响。添加的分子,主要是锂离子载体V,也称为12-crown-4,对锂具有很高的亲和力,因此破坏了锂阳离子与阴离子的偶联,从而显着提高了转移数。首先,我们通过研究它们的簇来研究锂-阴离子的空间相关性,并表明12冠4醚可以形成以前不存在的带正电的含锂配合物。然后,我们证明了当将离子载体分子添加到系统中时,给定阴离子配位笼的持续时间会减少,从而螯合剂与阴离子积极竞争来配位锂离子。最后,我们报告了随着添加的12-crown-4(和另一个离子载体,18-crown-6)分子的作用,各种化学物质的锂转移数增加,甚至可以达到正值。我们的结果为新设计和优化策略提供了基础,该策略可以逆转高度相关的浓缩电解质的符号并增加其迁移数。我们报告说,随着添加的12-crown-4(和另一个离子载体,18-crown-6)分子的作用,各种化学物质的锂转移数增加,甚至可以达到正值。我们的结果为新设计和优化策略提供了基础,该策略可以逆转高度相关的浓缩电解质的符号并增加其迁移数。我们报告说,随着添加的12-crown-4(和另一个离子载体,18-crown-6)分子的作用,各种化学物质的锂转移数增加,甚至可以达到正值。我们的结果为新设计和优化策略提供了基础,该策略可以逆转高度相关的浓缩电解质的符号并增加其迁移数。



























 京公网安备 11010802027423号
京公网安备 11010802027423号