当前位置:
X-MOL 学术
›
ChemElectroChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Recent Enzymatic Electrochemistry for Reductive Reactions
ChemElectroChem ( IF 4 ) Pub Date : 2020-04-02 , DOI: 10.1002/celc.202000282 Cécile Cadoux 1 , Ross D. Milton 1
ChemElectroChem ( IF 4 ) Pub Date : 2020-04-02 , DOI: 10.1002/celc.202000282 Cécile Cadoux 1 , Ross D. Milton 1
Affiliation
|
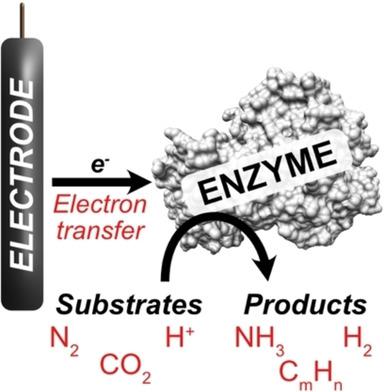
Enzymatic electrochemistry is the coupling of oxidoreductase enzymes to electrodes, where electrons are transferred between the electrode and an enzyme's cofactor(s). In addition to enzymatic electrochemistry enabling mechanistic study [such as the determination of cofactor reduction potential(s)], enzymatic electrocatalysis also enables substrate reduction or oxidation by exploiting the catalytic properties of enzymes. This Minireview illustrates some recent examples, in which electrodes are coupled with enzymes that catalyze the reduction of substrates such as dinitrogen (N2), carbon dioxide (CO2) and protons (H+), performed by metalloenzymes such as nitrogenases, formate dehydrogenases and hydrogenases. We review some strategies to achieve electron transfer (such as mediated and direct electron transfer), as well as some key results of recent studies.
中文翻译:

近期用于还原反应的酶电化学
酶促电化学是氧化还原酶与电极的偶联,其中电子在电极和酶的辅因子之间转移。除了可以进行机理研究[例如确定辅因子还原电位的方法]的酶促电化学以外,酶促电催化还可以通过利用酶的催化特性来实现底物的还原或氧化。本微型评论说明了一些最近的示例,其中电极与催化底物还原的酶结合,例如二氮(N 2),二氧化碳(CO 2)和质子(H +),由金属酶(如固氮酶,甲酸盐脱氢酶和氢化酶)进行。我们回顾了实现电子转移的一些策略(例如介导的和直接的电子转移),以及近期研究的一些关键成果。
更新日期:2020-04-02
中文翻译:

近期用于还原反应的酶电化学
酶促电化学是氧化还原酶与电极的偶联,其中电子在电极和酶的辅因子之间转移。除了可以进行机理研究[例如确定辅因子还原电位的方法]的酶促电化学以外,酶促电催化还可以通过利用酶的催化特性来实现底物的还原或氧化。本微型评论说明了一些最近的示例,其中电极与催化底物还原的酶结合,例如二氮(N 2),二氧化碳(CO 2)和质子(H +),由金属酶(如固氮酶,甲酸盐脱氢酶和氢化酶)进行。我们回顾了实现电子转移的一些策略(例如介导的和直接的电子转移),以及近期研究的一些关键成果。


























 京公网安备 11010802027423号
京公网安备 11010802027423号