JAMA Oncology ( IF 28.4 ) Pub Date : 2020-05-01 , DOI: 10.1001/jamaoncol.2020.0007 Esther Pohl-Rescigno 1 , Jan Hauke 1 , Sibylle Loibl 2 , Volker Möbus 3 , Carsten Denkert 4 , Peter A Fasching 5 , Mohamad Kayali 1 , Corinna Ernst 1 , Nana Weber-Lassalle 1 , Claus Hanusch 6 , Hans Tesch 7 , Volkmar Müller 8 , Janine Altmüller 9, 10 , Holger Thiele 9 , Michael Untch 11 , Kristina Lübbe 12 , Peter Nürnberg 9, 10, 13 , Kerstin Rhiem 1 , Jenny Furlanetto 2 , Bianca Lederer 2 , Christian Jackisch 14 , Valentina Nekljudova 2 , Rita K Schmutzler 1 , Andreas Schneeweiss 15 , Eric Hahnen 1
|
Importance The GeparOcto randomized clinical trial compared the efficacy of 2 neoadjuvant breast cancer (BC) treatment regimens: sequential intense dose-dense epirubicin, paclitaxel, and cyclophosphamide (iddEPC) vs weekly paclitaxel and nonpegylated liposomal doxorubicin (PM) in patients with different biological BC subtypes. Patients with triple-negative BC (TNBC) randomized to the PM arm received additional carboplatin (PMCb). Overall, no difference in pathologic complete response (pCR) rates was observed between study arms. It remained elusive whether the germline variant status of BRCA1/2 and further BC predisposition genes are associated with treatment outcome.
Objective To determine treatment outcome for BC according to germline variant status.
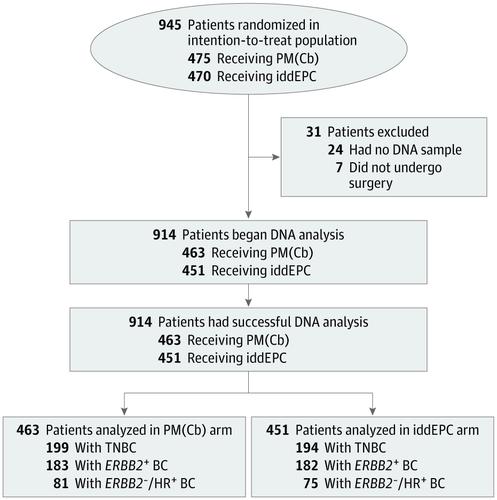
Design, Setting, and Participants This retrospective biomarker study is a secondary analysis of the GeparOcto multicenter prospective randomized clinical trial conducted between December 2014 and June 2016. Genetic analyses assessing for variants in BRCA1/2 and 16 other BC predisposition genes in 914 of 945 women were performed at the Center for Familial Breast and Ovarian Cancer, Cologne, Germany, from August 2017 through December 2018.
Main Outcomes and Measures Proportion of patients who achieved pCR (ypT0/is ypN0 definition) after neoadjuvant treatment according to germline variant status.
Results In the study sample of 914 women with different BC subtypes with a mean (range) age at BC diagnosis of 48 (21-76) years, overall higher pCR rates were observed in patients with BRCA1/2 variants than in patients without (60.4% vs 46.7%; odds ratio [OR], 1.74; 95% CI, 1.13-2.68; P = .01); variants in non-BRCA1/2 BC predisposition genes were not associated with therapy response. Patients with TNBC with BRCA1/2 variants achieved highest pCR rates. In the TNBC subgroup, a positive BRCA1/2 variant status was associated with therapy response in both the PMCb arm (74.3% vs 47.0% without BRCA1/2 variant; OR, 3.26; 95% CI, 1.44-7.39; P = .005) and the iddEPC arm (64.7% vs 45.0%; OR, 2.24; 95% CI, 1.04-4.84; P = .04). A positive BRCA1/2 variant status was also associated with elevated pCR rates in patients with ERBB2-negative, hormone receptor–positive BC (31.8% vs 11.9%; OR, 3.44; 95% CI, 1.22-9.72; P = .02).
Conclusions and Relevance Effective chemotherapy for BRCA1/2-mutated TNBC is commonly suggested to be platinum based. With a pCR rate of 64.7%, iddEPC may also be effective in these patients, though further prospective studies are needed. The elevated pCR rate in BRCA1/2-mutated ERBB2-negative, hormone receptor–positive BC suggests that germline BRCA1/2 testing should be considered prior to treatment start.
Trial Registration ClinicalTrials.gov Identifier: NCT02125344
中文翻译:

高危早期乳腺癌中生殖力变异状态与治疗反应的关联:GeparOcto随机临床试验的次要分析。
重要性 GeparOcto随机临床试验比较了两种新辅助乳腺癌(BC)治疗方案的疗效:序贯强剂量密集表柔比星,紫杉醇和环磷酰胺(iddEPC)与每周紫杉醇和非聚乙二醇脂质体阿霉素(PM)的比较亚型。随机分配至PM组的三阴性BC(TNBC)患者接受了额外的卡铂(PMCb)。总体而言,研究组之间未观察到病理完全缓解(pCR)率的差异。尚不清楚BRCA1 / 2的种系变异状态和其他BC易感基因是否与治疗结果相关。
目的 根据种系变异状况确定BC的治疗结果。
设计,背景和参与者 这项回顾性生物标志物研究是对2014年12月至2016年6月间进行的GeparOcto多中心前瞻性随机临床试验的二次分析。遗传分析评估了945名女性中的914名中的BRCA1 / 2和其他16个BC易感基因。该研究于2017年8月至2018年12月在德国科隆家族性乳腺癌和卵巢癌中心进行。
主要结果和措施 根据种系变异状态,在新辅助治疗后达到pCR(ypT0 /是ypN0定义)的患者比例。
结果 在对914位不同BC亚型,平均(范围)BC诊断为48(21-76)岁的女性的研究样本中,发现BRCA1 / 2变异患者的总体pCR率高于未感染(60.4)的患者。 %vs 46.7%;优势比[OR]为1.74; 95%CI为1.13-2.68;P = 0.01);非BRCA1 / 2 BC易感基因的变异与治疗反应无关。患有BRCA1 / 2变异的TNBC患者获得了最高的pCR率。在TNBC亚组中,两个PMCb组的BRCA1 / 2变异阳性状态均与治疗反应相关(74.3%vs无BRCA1 / 2变异的47.0%; OR为3.26; 95%CI为1.44-7.39;P = .005)和iddEPC组(64.7%对45.0%; OR为2.24; 95%CI为1.04-4.84;P = .04)。正的BRCA1 / 2变体状态也与pCR率升高相关联的患者的ERBB2阴性,激素受体阳性BC(31.8%对11.9%; OR,3.44; 95%CI,1.22-9.72; P = 0.02) 。
结论和相关性 通常建议对BRCA1 / 2突变的TNBC进行有效的化疗是基于铂的。尽管需要进一步的前瞻性研究,但iddEPC的pCR率为64.7%,对这些患者也可能有效。在BRCA1 / 2突变的ERBB2阴性,激素受体阳性的BC中pCR升高,提示在开始治疗之前应考虑种系BRCA1 / 2检测。
试验注册 ClinicalTrials.gov标识符:NCT02125344


























 京公网安备 11010802027423号
京公网安备 11010802027423号