Chemical Engineering Research and Design ( IF 3.9 ) Pub Date : 2020-03-12 , DOI: 10.1016/j.cherd.2020.02.020 Yuting Chu , Muhammad Asim Khan , Mingzhu Xia , Wu Lei , Fengyun Wang , Sidi Zhu , Xin Yan
|
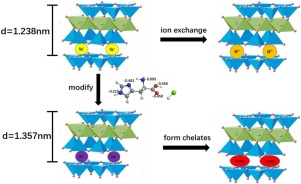
A novel nanocomposite of sodium montmorillonite with L-Histidine (His-Mt), was prepared to remove Pb(II) and Cu(II) from aqueous media. The resulting nanocomposites were characterized by XRD, FT-IR, BET, SEM, TG/DTG, XPS techniques. All characterizations confirmed the anchoring of L-Histidine to Na-Mt, and the packing sequence of His in the interlayer gallery may form a monolayer structure. The His salt obtained a skewed orientation compared to the silicate surface. The single/ co-adsorption of Cu(II) and Pb(II) by pure and modified i.e. Na-Mt and His-Mt were carried out by batch adsorption experiments. The maximum adsorption capacity (qmax) of Pb(II) and Cu(II) by Na-Mt and modified His-Mt was 89.08 mg/g, 23.93 mg/g, and 107.73 mg/g, 30.72 mg/g, correspondingly. The pseudo-second-order model (kinetic model) and Langmuir model (isotherm model) well-matched with the adsorption process. The data analysis showed it was an endothermic, favorable and spontaneous process, Cu(II) and Pb(II) showed competitive adsorption in the co-adsorption system. Na-Mt adsorbed M(II) ions by ion exchange mechanism, while M(II) formed complexes with histidine and were adsorbed in the interlayer region or the surface of His-Mt.
中文翻译:

组氨酸修饰的蒙脱土对废水中铅(II)和铜(II)的吸附作用及其合成与微观机理研究
制备了一种新型的蒙脱土钠与L-组氨酸(His-Mt)的纳米复合材料,用于从水性介质中去除Pb(II)和Cu(II)。通过XRD,FT-IR,BET,SEM,TG / DTG,XPS技术对所得纳米复合材料进行表征。所有特征都证实了L-组氨酸锚定在Na-Mt上,His在层间通道中的堆积顺序可能形成单层结构。与硅酸盐表面相比,His盐具有偏斜的取向。通过分批吸附实验对Cu(II)和Pb(II)进行纯净或修饰的Na-Mt和His-Mt单一/共吸附。最大吸附量(q maxNa-Mt和修饰的His-Mt对Pb(II)和Cu(II)的吸附量分别为89.08 mg / g,23.93 mg / g和107.73 mg / g,30.72 mg / g。伪二级模型(动力学模型)和Langmuir模型(等温模型)与吸附过程完全匹配。数据分析表明这是一个吸热,有利和自发的过程,Cu(II)和Pb(II)在共吸附体系中表现出竞争性吸附。Na-Mt通过离子交换机制吸附M(II)离子,而M(II)与组氨酸形成络合物,并被吸附在His-Mt的夹层区域或表面。



























 京公网安备 11010802027423号
京公网安备 11010802027423号