当前位置:
X-MOL 学术
›
Electroanalysis
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Electrochemical Conversion of Magnetic Nanoparticles Using Disposable Working Electrode in a 3D‐Printed Electrochemical Cell
Electroanalysis ( IF 3 ) Pub Date : 2020-02-27 , DOI: 10.1002/elan.202000035 Yawen He 1 , Fei Jia 1 , Junfei Guan 1 , Yingchun Fu 1 , Yanbin Li 1, 2
Electroanalysis ( IF 3 ) Pub Date : 2020-02-27 , DOI: 10.1002/elan.202000035 Yawen He 1 , Fei Jia 1 , Junfei Guan 1 , Yingchun Fu 1 , Yanbin Li 1, 2
Affiliation
|
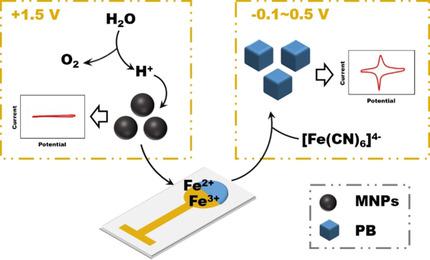
Exploration of new property/function of nanomaterials is always a strong impetus in the nanoscience field. Here, a new method of electrochemical conversion (ECC) of magnetic nanoparticles (MNPs) is proposed to endow MNPs with signal generation ability for sensing. Briefly, high potential was applied to split H2O to generate acid, while Fe3O4 MNPs reacted with H+ and produce ferric/ferrous ions, which further reacted with K4Fe(CN)6 to yield Prussian blue (PB) through potential cycling. The ECC method worked well on both home‐made and commercial MNPs with different sizes. The generated PB possessed strong electrochemical activity for further applications. Interestingly, an uneven deposition of PB on working electrode and undesired contamination of the reference and counter electrodes were found when using commercial integrated three‐electrode chip. A 3D‐printed electrochemical cell was designed to facilitate the ECC and avoid drawbacks of commercial integrated electrode. The 3D‐printed electrochemical cell was proven to solve the problem above through spatial separation of electrodes and thus facilitated the ECC process. An electrochemical sensor for H2O2 detection based on the catalysis ability of ECC‐based PB exhibited a linear response from 5 μM to 1 mM, a high sensitivity of 269 μA mM−1 cm−2 and a low detection limit of 0.16 μM (S/N =3), which suggests its promising application prospect in electrochemistry‐related analysis.
中文翻译:

使用3D打印电化学池中的一次性工作电极对磁性纳米粒子进行电化学转化
探索纳米材料的新特性/功能始终是纳米科学领域的强大动力。在此,提出了一种磁性纳米粒子(MNP)电化学转化(ECC)的新方法,以赋予MNP感应信号的能力。简而言之,将高电势用于分解H 2 O生成酸,而Fe 3 O 4 MNP与H +反应并生成铁/亚铁离子,然后进一步与K 4 Fe(CN)6反应通过潜在的循环产生普鲁士蓝(PB)。ECC方法在大小不同的自制和商用MNP上均能很好地工作。生成的PB具有很强的电化学活性,可用于进一步的应用。有趣的是,使用商用集成式三电极芯片时,发现PB在工作电极上的沉积不均匀,以及参比电极和对电极受到不希望的污染。设计了3D打印的电化学电池,以促进ECC并避免商业集成电极的缺点。经证明,3D打印的电化学电池通过电极的空间分隔解决了上述问题,从而简化了ECC过程。H 2 O 2电化学传感器基于ECC的PB的催化能力进行的检测显示出从5μM到1 mM的线性响应,269μAmM -1 cm -2的高灵敏度和0.16μM的低检测限(S / N = 3),这表明它在电化学相关分析中具有广阔的应用前景。
更新日期:2020-02-27
中文翻译:

使用3D打印电化学池中的一次性工作电极对磁性纳米粒子进行电化学转化
探索纳米材料的新特性/功能始终是纳米科学领域的强大动力。在此,提出了一种磁性纳米粒子(MNP)电化学转化(ECC)的新方法,以赋予MNP感应信号的能力。简而言之,将高电势用于分解H 2 O生成酸,而Fe 3 O 4 MNP与H +反应并生成铁/亚铁离子,然后进一步与K 4 Fe(CN)6反应通过潜在的循环产生普鲁士蓝(PB)。ECC方法在大小不同的自制和商用MNP上均能很好地工作。生成的PB具有很强的电化学活性,可用于进一步的应用。有趣的是,使用商用集成式三电极芯片时,发现PB在工作电极上的沉积不均匀,以及参比电极和对电极受到不希望的污染。设计了3D打印的电化学电池,以促进ECC并避免商业集成电极的缺点。经证明,3D打印的电化学电池通过电极的空间分隔解决了上述问题,从而简化了ECC过程。H 2 O 2电化学传感器基于ECC的PB的催化能力进行的检测显示出从5μM到1 mM的线性响应,269μAmM -1 cm -2的高灵敏度和0.16μM的低检测限(S / N = 3),这表明它在电化学相关分析中具有广阔的应用前景。



























 京公网安备 11010802027423号
京公网安备 11010802027423号