当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Tandem sequential catalytic enantioselective synthesis of highly-functionalised tetrahydroindolizine derivatives
Chemical Science ( IF 8.4 ) Pub Date : 2020-03-12 , DOI: 10.1039/d0sc00432d Shuyue Zhang 1, 2, 3, 4, 5 , Mark D. Greenhalgh 1, 2, 3, 4, 5 , Alexandra M. Z. Slawin 1, 2, 3, 4, 5 , Andrew D. Smith 1, 2, 3, 4, 5
Chemical Science ( IF 8.4 ) Pub Date : 2020-03-12 , DOI: 10.1039/d0sc00432d Shuyue Zhang 1, 2, 3, 4, 5 , Mark D. Greenhalgh 1, 2, 3, 4, 5 , Alexandra M. Z. Slawin 1, 2, 3, 4, 5 , Andrew D. Smith 1, 2, 3, 4, 5
Affiliation
|
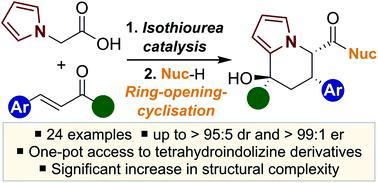
An isothiourea-catalysed enantioselective synthesis of novel tetrahydroindolizine derivatives is reported through a one-pot tandem sequential process. The application of 2-(pyrrol-1-yl)acetic acid in combination with either a trifluoromethyl enone or an α-keto-β,γ-unsaturated ester in an enantioselective Michael addition–lactonisation process, followed by in situ ring-opening and cyclisation, led to a range of 24 tetrahydroindolizine derivatives containing three stereocentres in up to >95 : 5 dr and >99 : 1 er.
中文翻译:

高功能化四氢吲哚嗪衍生物的串联顺序催化对映选择性合成
通过一锅串联顺序过程报道了新型四氢吲哚嗪衍生物的异硫脲催化的对映选择性合成。在对映选择性迈克尔加成-内酯化过程中,将2-(吡咯-1-基)乙酸与三氟甲基烯酮或α-酮-β,γ-不饱和酯结合使用,然后进行原位开环和环化,导致一系列24种四氢吲哚嗪衍生物包含三个立体中心,其最大> 95:5 dr和> 99:1 er。
更新日期:2020-04-24
中文翻译:

高功能化四氢吲哚嗪衍生物的串联顺序催化对映选择性合成
通过一锅串联顺序过程报道了新型四氢吲哚嗪衍生物的异硫脲催化的对映选择性合成。在对映选择性迈克尔加成-内酯化过程中,将2-(吡咯-1-基)乙酸与三氟甲基烯酮或α-酮-β,γ-不饱和酯结合使用,然后进行原位开环和环化,导致一系列24种四氢吲哚嗪衍生物包含三个立体中心,其最大> 95:5 dr和> 99:1 er。



























 京公网安备 11010802027423号
京公网安备 11010802027423号