当前位置:
X-MOL 学术
›
React. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Experimental and computational investigations of C–H activation of cyclohexane by ozone in liquid CO2
Reaction Chemistry & Engineering ( IF 3.9 ) Pub Date : 2020-03-11 , DOI: 10.1039/c9re00442d Xuhui Chen 1, 2, 3, 4, 5 , Derek B. Rice 1, 2, 3, 4, 6 , Andrew M. Danby 1, 2, 3, 4, 5 , Michael D. Lundin 1, 2, 3, 4, 5 , Timothy A. Jackson 1, 2, 3, 4, 6 , Bala Subramaniam 1, 2, 3, 4, 5
Reaction Chemistry & Engineering ( IF 3.9 ) Pub Date : 2020-03-11 , DOI: 10.1039/c9re00442d Xuhui Chen 1, 2, 3, 4, 5 , Derek B. Rice 1, 2, 3, 4, 6 , Andrew M. Danby 1, 2, 3, 4, 5 , Michael D. Lundin 1, 2, 3, 4, 5 , Timothy A. Jackson 1, 2, 3, 4, 6 , Bala Subramaniam 1, 2, 3, 4, 5
Affiliation
|
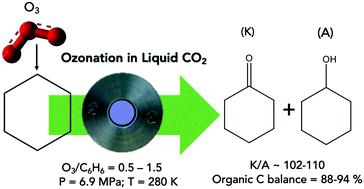
Facile cyclohexane oxidation by ozone in liquid CO2 was demonstrated in a Parr reactor equipped with in situ infrared probe. The dominant presence of CO2 in the vapor phase allows operation below the lower flammability limit. At 280 K, 6.9 MPa and a molar feed O3/cyclohexane ratio ≃0.5, the cyclohexane conversion was ∼12% during a 1 h batch run with cyclohexanone (K) + cyclohexanol (A) yield and K/A being ∼11% and ∼100, respectively. Small but measurable amounts of hydrogen peroxide were also formed. The absence of any detectable water in the product and the close match of cyclohexane conversion and product yield rule out significant cyclohexane combustion. The observed products are consistent with reaction pathways predicted by density functional theory (DFT) computations and involve the formation of a hydrotrioxide (HO3C6H11) intermediate. The DFT-computed energetics of the initial hydrogen-atom transfer reaction between O3 and cyclohexane to give the hydrotrioxide were validated by comparison with multi-reference complete active space self-consistent field (CASSCF) computations, which included n-electron valence state perturbation theory (NEVPT2) for dynamic correlation. Even though DFT cannot properly treat the multireference character of O3, the agreement between these methods was reasonable. Preliminary studies of isooctane ozonation demonstrate that ozone is capable of activating all carbons (with a preference for the primary carbon) producing a variety of oxygenated products (alcohols, ketones and acid) with negligible formation of combustion products. These results clearly demonstrate the ability of ozone in selectively oxidizing C–H bonds in alkanes.
中文翻译:

液态二氧化碳中臭氧对C–H活化环己烷的实验和计算研究
在配备有原位红外探针的Parr反应器中证明了在液态CO 2中臭氧容易进行的环己烷氧化。气相中CO 2的主要存在允许在较低的可燃性极限以下运行。在280 K,6.9 MPa和摩尔进料为O 3的情况下/环己烷比率≃0.5,在1 h批处理运行中,环己酮(K)+环己醇(A)的产率为〜12%,K / A分别为〜11%和〜100。还形成了少量但可测量的过氧化氢。产物中没有任何可检测到的水,并且环己烷转化率和产物产率紧密匹配,排除了明显的环己烷燃烧。观察到的产物与通过密度泛函理论(DFT)计算预测的反应路径一致,并且涉及形成氢三氧化物(HO 3 C 6 H 11)中间体。D 3计算的O 3之间初始氢原子转移反应的能量学通过与多参比完整活性空间自洽场(CASSCF)计算进行比较,验证了环己烷和环己烷生成的氢氧化合物,该计算包括用于动态相关的n电子价态扰动理论(NEVPT2)。即使DFT无法正确处理O 3的多引用特征,这些方法之间的协议还是合理的。异辛烷臭氧化的初步研究表明,臭氧能够活化所有碳(优先选择伯碳),从而产生各种氧化产物(醇,酮和酸),而燃烧产物的形成可以忽略不计。这些结果清楚地证明了臭氧具有选择性氧化烷烃中C–H键的能力。
更新日期:2020-04-24
中文翻译:

液态二氧化碳中臭氧对C–H活化环己烷的实验和计算研究
在配备有原位红外探针的Parr反应器中证明了在液态CO 2中臭氧容易进行的环己烷氧化。气相中CO 2的主要存在允许在较低的可燃性极限以下运行。在280 K,6.9 MPa和摩尔进料为O 3的情况下/环己烷比率≃0.5,在1 h批处理运行中,环己酮(K)+环己醇(A)的产率为〜12%,K / A分别为〜11%和〜100。还形成了少量但可测量的过氧化氢。产物中没有任何可检测到的水,并且环己烷转化率和产物产率紧密匹配,排除了明显的环己烷燃烧。观察到的产物与通过密度泛函理论(DFT)计算预测的反应路径一致,并且涉及形成氢三氧化物(HO 3 C 6 H 11)中间体。D 3计算的O 3之间初始氢原子转移反应的能量学通过与多参比完整活性空间自洽场(CASSCF)计算进行比较,验证了环己烷和环己烷生成的氢氧化合物,该计算包括用于动态相关的n电子价态扰动理论(NEVPT2)。即使DFT无法正确处理O 3的多引用特征,这些方法之间的协议还是合理的。异辛烷臭氧化的初步研究表明,臭氧能够活化所有碳(优先选择伯碳),从而产生各种氧化产物(醇,酮和酸),而燃烧产物的形成可以忽略不计。这些结果清楚地证明了臭氧具有选择性氧化烷烃中C–H键的能力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号