Molecular Psychiatry ( IF 11.0 ) Pub Date : 2020-03-11 , DOI: 10.1038/s41380-020-0708-6 Farhan Ali 1 , Ling-Xiao Shao 1 , Danielle M Gerhard 1 , Katherine Sweasy 1 , Santosh Pothula 1 , Christopher Pittenger 1, 2 , Ronald S Duman 1, 3 , Alex C Kwan 1, 3
|
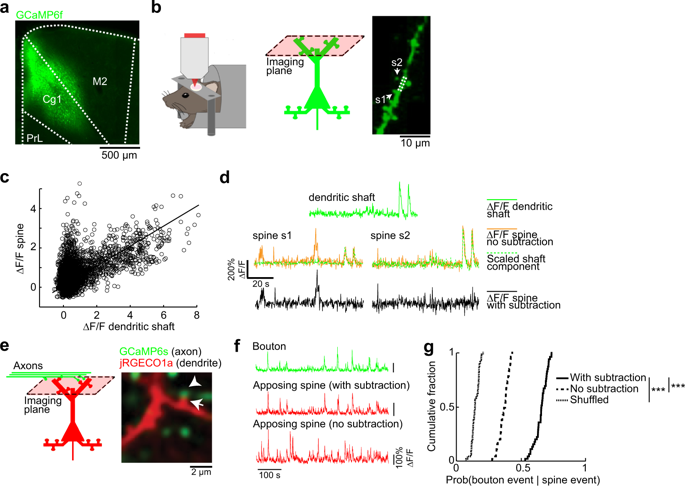
The SHANK3 gene encodes a postsynaptic scaffold protein in excitatory synapses, and its disruption is implicated in neurodevelopmental disorders such as Phelan–McDermid syndrome, autism spectrum disorder, and schizophrenia. Most studies of SHANK3 in the neocortex and hippocampus have focused on disturbances in pyramidal neurons. However, GABAergic interneurons likewise receive excitatory inputs and presumably would also be a target of constitutive SHANK3 perturbations. In this study, we characterize the prefrontal cortical microcircuit in awake mice using subcellular-resolution two-photon microscopy. We focused on a nonsense R1117X mutation, which leads to truncated SHANK3 and has been linked previously to cortical dysfunction. We find that R1117X mutants have abnormally elevated calcium transients in apical dendritic spines. The synaptic calcium dysregulation is due to a loss of dendritic inhibition via decreased NMDAR currents and reduced firing of dendrite-targeting somatostatin-expressing (SST) GABAergic interneurons. Notably, upregulation of the NMDAR subunit GluN2B in SST interneurons corrects the excessive synaptic calcium signals and ameliorates learning deficits in R1117X mutants. These findings reveal dendrite-targeting interneurons, and more broadly the inhibitory control of dendritic spines, as a key microcircuit mechanism compromised by the SHANK3 dysfunction.
中文翻译:

无意义的 Shank3 突变损害了前额树突棘中钙瞬变的抑制性调节。
SHANK3 _基因编码兴奋性突触中的突触后支架蛋白,其破坏与神经发育障碍有关,例如 Phelan-McDermid 综合征、自闭症谱系障碍和精神分裂症。大多数关于新皮质和海马中 SHANK3 的研究都集中在锥体神经元的紊乱上。然而,GABAergic 中间神经元同样接受兴奋性输入,并且可能也是本构 SHANK3 扰动的目标。在这项研究中,我们使用亚细胞分辨率双光子显微镜对清醒小鼠的前额皮质微电路进行了表征。我们专注于一个无意义的 R1117X 突变,它导致 SHANK3 被截断,并且以前与皮质功能障碍有关。我们发现 R1117X 突变体在顶端树突棘中钙瞬变异常升高。突触钙失调是由于通过减少 NMDAR 电流和减少树突靶向生长抑素表达 (SST) GABAergic 中间神经元的放电而丧失树突抑制。值得注意的是,SST 中间神经元中 NMDAR 亚基 GluN2B 的上调可纠正过度的突触钙信号并改善 R1117X 突变体的学习缺陷。这些发现揭示了树突靶向中间神经元,更广泛地说,树突棘的抑制控制是受 SHANK3 功能障碍影响的关键微电路机制。SST 中间神经元中 NMDAR 亚基 GluN2B 的上调可纠正过度的突触钙信号并改善 R1117X 突变体的学习缺陷。这些发现揭示了树突靶向中间神经元,更广泛地说,树突棘的抑制控制是受 SHANK3 功能障碍影响的关键微电路机制。SST 中间神经元中 NMDAR 亚基 GluN2B 的上调可纠正过度的突触钙信号并改善 R1117X 突变体的学习缺陷。这些发现揭示了树突靶向中间神经元,更广泛地说,树突棘的抑制控制是受 SHANK3 功能障碍影响的关键微电路机制。



























 京公网安备 11010802027423号
京公网安备 11010802027423号