当前位置:
X-MOL 学术
›
Br. J. Cancer
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Two first-in-human studies of xentuzumab, a humanised insulin-like growth factor (IGF)-neutralising antibody, in patients with advanced solid tumours.
British Journal of Cancer ( IF 8.8 ) Pub Date : 2020-03-12 , DOI: 10.1038/s41416-020-0774-1 Johann de Bono , Chia-Chi Lin , Li-Tzong Chen , Jesus Corral , Vasiliki Michalarea , Karim Rihawi , Michael Ong , Jih-Hsiang Lee , Chih-Hung Hsu , James Chih-Hsin Yang , Her-Shyong Shiah , Chia-Jui Yen , Alan Anthoney , Maria Jove , Susanne Buschke , René Fuertig , Ulrike Schmid , Rainer-Georg Goeldner , Natalja Strelkowa , Dennis Chin-Lun Huang , Thomas Bogenrieder , Chris Twelves , Ann-Lii Cheng
British Journal of Cancer ( IF 8.8 ) Pub Date : 2020-03-12 , DOI: 10.1038/s41416-020-0774-1 Johann de Bono , Chia-Chi Lin , Li-Tzong Chen , Jesus Corral , Vasiliki Michalarea , Karim Rihawi , Michael Ong , Jih-Hsiang Lee , Chih-Hung Hsu , James Chih-Hsin Yang , Her-Shyong Shiah , Chia-Jui Yen , Alan Anthoney , Maria Jove , Susanne Buschke , René Fuertig , Ulrike Schmid , Rainer-Georg Goeldner , Natalja Strelkowa , Dennis Chin-Lun Huang , Thomas Bogenrieder , Chris Twelves , Ann-Lii Cheng
|
BACKGROUND
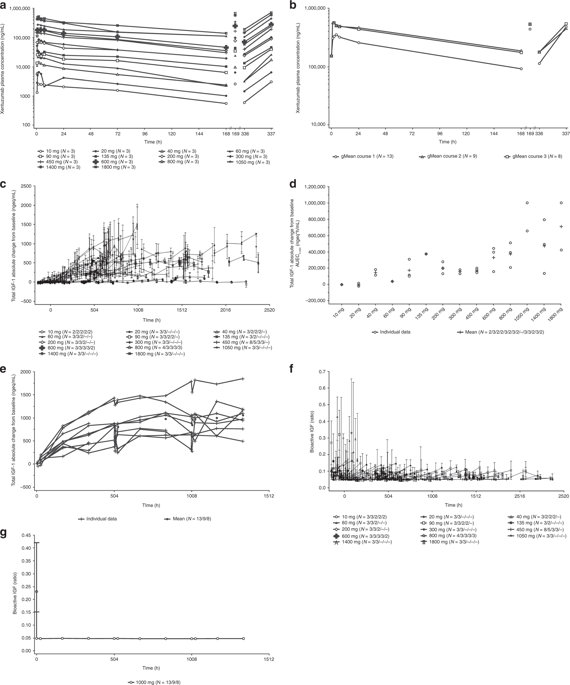
Xentuzumab, an insulin-like growth factor (IGF)-1/IGF-2-neutralising antibody, binds IGF-1 and IGF-2, inhibiting their growth-promoting signalling. Two first-in-human trials assessed the maximum-tolerated/relevant biological dose (MTD/RBD), safety, pharmacokinetics, pharmacodynamics, and activity of xentuzumab in advanced/metastatic solid cancers.
METHODS
These phase 1, open-label trials comprised dose-finding (part I; 3 + 3 design) and expansion cohorts (part II; selected tumours; RBD [weekly dosing]). Primary endpoints were MTD/RBD.
RESULTS
Study 1280.1 involved 61 patients (part I: xentuzumab 10-1800 mg weekly, n = 48; part II: 1000 mg weekly, n = 13); study 1280.2, 64 patients (part I: 10-3600 mg three-weekly, n = 33; part II: 1000 mg weekly, n = 31). One dose-limiting toxicity occurred; the MTD was not reached for either schedule. Adverse events were generally grade 1/2, mostly gastrointestinal. Xentuzumab showed dose-proportional pharmacokinetics. Total plasma IGF-1 increased dose dependently, plateauing at ~1000 mg/week; at ≥450 mg/week, IGF bioactivity was almost undetectable. Two partial responses occurred (poorly differentiated nasopharyngeal carcinoma and peripheral primitive neuroectodermal tumour). Integration of biomarker and response data by Bayesian Logistic Regression Modeling (BLRM) confirmed the RBD.
CONCLUSIONS
Xentuzumab was well tolerated; MTD was not reached. RBD was 1000 mg weekly, confirmed by BLRM. Xentuzumab showed preliminary anti-tumour activity.
CLINICAL TRIAL REGISTRATION
NCT01403974; NCT01317420.
中文翻译:

xentuzumab 是一种人源化胰岛素样生长因子 (IGF) 中和抗体,针对晚期实体瘤患者进行了两项首次人体研究。
背景 Xentuzumab 是一种胰岛素样生长因子 (IGF)-1/IGF-2 中和抗体,可结合 IGF-1 和 IGF-2,抑制其生长促进信号传导。两项首次人体试验评估了 Xentuzumab 在晚期/转移性实体癌中的最大耐受/相关生物剂量 (MTD/RBD)、安全性、药代动力学、药效学和活性。方法 这些 1 期开放标签试验包括剂量探索(第一部分;3 + 3 设计)和扩展队列(第二部分;选定的肿瘤;RBD [每周给药])。主要终点是 MTD/RBD。结果 研究 1280.1 涉及 61 名患者(第一部分:xentuzumab 每周 10-1800 mg,n = 48;第二部分:每周 1000 mg,n = 13);研究 1280.2,64 名患者(第一部分:每三周 10-3600 毫克,n = 33;第二部分:每周 1000 毫克,n = 31)。发生一种剂量限制性毒性;两个计划均未达到 MTD。不良事件一般为1/2级,主要是胃肠道不良事件。Xentuzumab 显示出与剂量成比例的药代动力学。血浆总 IGF-1 呈剂量依赖性增加,稳定在约 1000 毫克/周;≥450 mg/周时,IGF 生物活性几乎检测不到。发生了两种部分反应(低分化鼻咽癌和周围原始神经外胚层肿瘤)。通过贝叶斯逻辑回归模型 (BLRM) 整合生物标志物和反应数据证实了 RBD。结论 Xentuzumab 耐受性良好;未达到 MTD。经 BLRM 确认,RBD 为每周 1000 毫克。Xentuzumab 显示出初步的抗肿瘤活性。临床试验注册NCT01403974;NCT01317420。
更新日期:2020-03-12
中文翻译:

xentuzumab 是一种人源化胰岛素样生长因子 (IGF) 中和抗体,针对晚期实体瘤患者进行了两项首次人体研究。
背景 Xentuzumab 是一种胰岛素样生长因子 (IGF)-1/IGF-2 中和抗体,可结合 IGF-1 和 IGF-2,抑制其生长促进信号传导。两项首次人体试验评估了 Xentuzumab 在晚期/转移性实体癌中的最大耐受/相关生物剂量 (MTD/RBD)、安全性、药代动力学、药效学和活性。方法 这些 1 期开放标签试验包括剂量探索(第一部分;3 + 3 设计)和扩展队列(第二部分;选定的肿瘤;RBD [每周给药])。主要终点是 MTD/RBD。结果 研究 1280.1 涉及 61 名患者(第一部分:xentuzumab 每周 10-1800 mg,n = 48;第二部分:每周 1000 mg,n = 13);研究 1280.2,64 名患者(第一部分:每三周 10-3600 毫克,n = 33;第二部分:每周 1000 毫克,n = 31)。发生一种剂量限制性毒性;两个计划均未达到 MTD。不良事件一般为1/2级,主要是胃肠道不良事件。Xentuzumab 显示出与剂量成比例的药代动力学。血浆总 IGF-1 呈剂量依赖性增加,稳定在约 1000 毫克/周;≥450 mg/周时,IGF 生物活性几乎检测不到。发生了两种部分反应(低分化鼻咽癌和周围原始神经外胚层肿瘤)。通过贝叶斯逻辑回归模型 (BLRM) 整合生物标志物和反应数据证实了 RBD。结论 Xentuzumab 耐受性良好;未达到 MTD。经 BLRM 确认,RBD 为每周 1000 毫克。Xentuzumab 显示出初步的抗肿瘤活性。临床试验注册NCT01403974;NCT01317420。

























 京公网安备 11010802027423号
京公网安备 11010802027423号