当前位置:
X-MOL 学术
›
ACS Cent. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Computational Design of an Allosteric Antibody Switch by Deletion and Rescue of a Complex Structural Constellation.
ACS Central Science ( IF 18.2 ) Pub Date : 2020-03-11 , DOI: 10.1021/acscentsci.9b01065 Jittasak Khowsathit 1, 2 , Andrea Bazzoli 2 , Hong Cheng 1 , John Karanicolas 1, 2, 2
ACS Central Science ( IF 18.2 ) Pub Date : 2020-03-11 , DOI: 10.1021/acscentsci.9b01065 Jittasak Khowsathit 1, 2 , Andrea Bazzoli 2 , Hong Cheng 1 , John Karanicolas 1, 2, 2
Affiliation
|
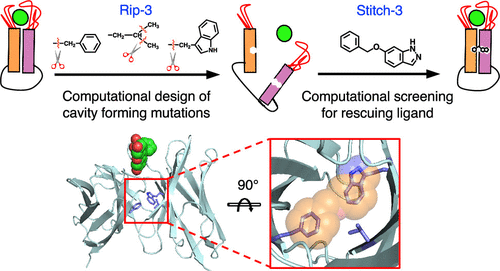
Therapeutic monoclonal antibodies have transformed medicine, especially with regards to treating cancers and disorders of the immune system. More than 50 antibody-derived drugs have already reached the clinic, the majority of which target cytokines or cell-surface receptors. Unfortunately, many of these targets have pleiotropic functions: they serve multiple different roles, and often not all of these roles are disease-related. This can be problematic because antibodies act throughout the body, and systemic neutralization of such targets can lead to safety concerns. To address this, we have developed a strategy whereby an antibody’s ability to recognize its antigen is modulated by a second layer of control, relying on addition of an exogenous small molecule. In previous studies, we began to explore this idea by introducing a deactivating tryptophan-to-glycine mutation in the domain–domain interface of a single-chain variable fragment (scFv), and then restoring activity by adding back indole to fit the designed cavity. Here, we now describe a novel computational strategy for enumerating larger cavities that can be formed by simultaneously introducing multiple adjacent large-to-small mutations; we then carry out a complementary virtual screen to identify druglike compounds to match each candidate cavity. We first demonstrate the utility of this strategy in a fluorescein-binding single-chain variable fragment (scFv) and experimentally characterize a triple mutant with reduced antigen-binding (Rip-3) that can be rescued using a complementary ligand (Stitch-3). Because our design is built upon conserved residues in the antibody framework, we then show that the same mutation/ligand pair can also be used to modulate antigen-binding in an scFv build from a completely unrelated framework. This set of residues is present in many therapeutic antibodies as well, suggesting that this mutation/ligand pair may serve as a general starting point for introducing ligand-dependence into many clinically relevant antibodies.
中文翻译:

通过删除和拯救复杂结构群来进行变构抗体开关的计算设计。
治疗性单克隆抗体已经改变了医学,特别是在治疗癌症和免疫系统疾病方面。超过 50 种抗体衍生药物已进入临床,其中大多数针对细胞因子或细胞表面受体。不幸的是,许多这些靶标具有多效性功能:它们具有多种不同的作用,而且通常并非所有这些作用都与疾病相关。这可能会产生问题,因为抗体在全身发挥作用,而这些靶标的全身中和可能会导致安全问题。为了解决这个问题,我们开发了一种策略,通过添加外源小分子,通过第二层控制来调节抗体识别其抗原的能力。在之前的研究中,我们开始探索这一想法,方法是在单链可变片段(scFv)的结构域-结构域界面中引入失活色氨酸到甘氨酸的突变,然后通过添加回吲哚以适应设计的空腔来恢复活性。在这里,我们现在描述了一种新的计算策略,用于枚举可以通过同时引入多个相邻的从大到小的突变来形成的较大空腔;然后,我们进行补充虚拟筛选,以识别与每个候选空腔相匹配的药物样化合物。我们首先在荧光素结合单链可变片段 (scFv) 中证明了该策略的实用性,并通过实验表征了抗原结合减少的三重突变体 (Rip-3),可以使用互补配体 (Stitch-3) 来挽救该突变体。因为我们的设计是建立在抗体框架中的保守残基的基础上,所以我们随后表明,相同的突变/配体对也可以用于调节从完全不相关的框架构建的 scFv 中的抗原结合。这组残基也存在于许多治疗性抗体中,表明该突变/配体对可以作为将配体依赖性引入许多临床相关抗体的一般起点。
更新日期:2020-03-26
中文翻译:

通过删除和拯救复杂结构群来进行变构抗体开关的计算设计。
治疗性单克隆抗体已经改变了医学,特别是在治疗癌症和免疫系统疾病方面。超过 50 种抗体衍生药物已进入临床,其中大多数针对细胞因子或细胞表面受体。不幸的是,许多这些靶标具有多效性功能:它们具有多种不同的作用,而且通常并非所有这些作用都与疾病相关。这可能会产生问题,因为抗体在全身发挥作用,而这些靶标的全身中和可能会导致安全问题。为了解决这个问题,我们开发了一种策略,通过添加外源小分子,通过第二层控制来调节抗体识别其抗原的能力。在之前的研究中,我们开始探索这一想法,方法是在单链可变片段(scFv)的结构域-结构域界面中引入失活色氨酸到甘氨酸的突变,然后通过添加回吲哚以适应设计的空腔来恢复活性。在这里,我们现在描述了一种新的计算策略,用于枚举可以通过同时引入多个相邻的从大到小的突变来形成的较大空腔;然后,我们进行补充虚拟筛选,以识别与每个候选空腔相匹配的药物样化合物。我们首先在荧光素结合单链可变片段 (scFv) 中证明了该策略的实用性,并通过实验表征了抗原结合减少的三重突变体 (Rip-3),可以使用互补配体 (Stitch-3) 来挽救该突变体。因为我们的设计是建立在抗体框架中的保守残基的基础上,所以我们随后表明,相同的突变/配体对也可以用于调节从完全不相关的框架构建的 scFv 中的抗原结合。这组残基也存在于许多治疗性抗体中,表明该突变/配体对可以作为将配体依赖性引入许多临床相关抗体的一般起点。


























 京公网安备 11010802027423号
京公网安备 11010802027423号