当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Computational Insights into the Divergent Regioselectivities for Nickel‐Catalyzed Dicarbofunctionalization of Allyl Moiety of N‐Allyl‐2‐aminopyrimidine
Asian Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2020-03-30 , DOI: 10.1002/ajoc.202000110 Guan‐Fu Yu 1 , Ping Wang 1 , Xiaoguang Bao 1 , Yong Wang 1
Asian Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2020-03-30 , DOI: 10.1002/ajoc.202000110 Guan‐Fu Yu 1 , Ping Wang 1 , Xiaoguang Bao 1 , Yong Wang 1
Affiliation
|
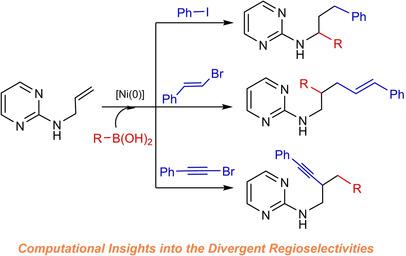
Herein, the origins of organohalide‐dependent regioselectivities for Ni‐catalyzed dicarbofunctionalization of allyl moiety of N‐allyl‐2‐aminopyrimidine were explored by computational studies. When aryl iodides are used for the dicarbofunctionalization, the detailed reaction mechanistic pathway follows the sequential oxidative addition (OA), migratory insertion (MI), β‐H elimination, H‐migration, transmetalation, and reductive elimination (RE) to afford the 1,3‐dicarbofunctionalization products. Due to the presence of chelation of the pyrimidinyl moiety of substrate with Ni, the competitive β‐H elimination leading to the product of Heck reaction is less favourable and thus the regioselective β‐H elimination occurs. The formation of thermodynamically more stable intermediate is the driving force for the subsequent H‐migration. When alkenyl bromides are used, alternative regioselectivity to give 1,2‐dicarbofunctionalization products is obtained and the proposed mechanistic pathway proceeds sequentially via OA, MI, transmetalation, and RE. The main reason accounting for the different regioselectivity between aryl iodides and alkenyl bromides involved in dicarbofunctionalization is revealed. In addition, the reversed 2,1‐regioselectivity achieved by using alkynyl halides in the Ni‐catalyzed dicarbofunctionalization is via sequential steps of OA, transmetalation, MI, and RE. The mechanistic difference that the migratory insertion step is unlikely to occur after OA, which is in contrast to the other two mechanistic pathways, is rationalized.
中文翻译:

N-烯丙基-2-氨基嘧啶的烯丙基部分镍催化双碳官能化的不同区域选择性的计算洞察
在本文中,Ni催化的N烯丙基部分的二碳官能化反应取决于有机卤化物的区域选择性通过计算研究探索了烯丙基-2-氨基嘧啶。当使用芳基碘化物进行双碳官能化时,详细的反应机理遵循顺序的氧化加成(OA),迁移插入(MI),β-H消除,H迁移,重金属化和还原消除(RE)来得到1 ,3-双线性功能化产品。由于底物的嘧啶基部分与Ni发生螯合,竞争性的β-H消除导致了Heck反应产物的产生不那么有利,因此发生了区域选择性的β-H消除。热力学上更稳定的中间体的形成是随后H迁移的驱动力。当使用烯基溴化物时,区域选择性选择性为1 获得了2-双线性官能化产物,并且所提出的机制途径依次通过OA,MI,过渡金属化和RE进行。揭示了解释参与双碳官能化的芳基碘化物和烯基溴化物之间区域选择性不同的主要原因。此外,在Ni催化的二碳官能化反应中使用卤化炔基可以逆转2,1-区域选择性,这是通过OA,过渡金属化,MI和RE的连续步骤完成的。与其他两种机理途径相反,合理化了OA后迁移插入步骤不太可能发生的机理差异。揭示了解释参与双碳官能化的芳基碘化物和烯基溴化物之间区域选择性不同的主要原因。此外,在Ni催化的双碳官能化反应中使用卤化炔基可以逆转2,1-区域选择性,这是通过OA,过渡金属化,MI和RE的连续步骤完成的。与其他两种机理途径相反,合理化了OA后迁移插入步骤不太可能发生的机理差异。揭示了解释参与双碳官能化的芳基碘化物和烯基溴化物之间区域选择性不同的主要原因。此外,在Ni催化的二碳官能化反应中使用卤化炔基可以逆转2,1-区域选择性,这是通过OA,过渡金属化,MI和RE的连续步骤完成的。与其他两种机理途径相反,合理化了OA后迁移插入步骤不太可能发生的机理差异。
更新日期:2020-03-30
中文翻译:

N-烯丙基-2-氨基嘧啶的烯丙基部分镍催化双碳官能化的不同区域选择性的计算洞察
在本文中,Ni催化的N烯丙基部分的二碳官能化反应取决于有机卤化物的区域选择性通过计算研究探索了烯丙基-2-氨基嘧啶。当使用芳基碘化物进行双碳官能化时,详细的反应机理遵循顺序的氧化加成(OA),迁移插入(MI),β-H消除,H迁移,重金属化和还原消除(RE)来得到1 ,3-双线性功能化产品。由于底物的嘧啶基部分与Ni发生螯合,竞争性的β-H消除导致了Heck反应产物的产生不那么有利,因此发生了区域选择性的β-H消除。热力学上更稳定的中间体的形成是随后H迁移的驱动力。当使用烯基溴化物时,区域选择性选择性为1 获得了2-双线性官能化产物,并且所提出的机制途径依次通过OA,MI,过渡金属化和RE进行。揭示了解释参与双碳官能化的芳基碘化物和烯基溴化物之间区域选择性不同的主要原因。此外,在Ni催化的二碳官能化反应中使用卤化炔基可以逆转2,1-区域选择性,这是通过OA,过渡金属化,MI和RE的连续步骤完成的。与其他两种机理途径相反,合理化了OA后迁移插入步骤不太可能发生的机理差异。揭示了解释参与双碳官能化的芳基碘化物和烯基溴化物之间区域选择性不同的主要原因。此外,在Ni催化的双碳官能化反应中使用卤化炔基可以逆转2,1-区域选择性,这是通过OA,过渡金属化,MI和RE的连续步骤完成的。与其他两种机理途径相反,合理化了OA后迁移插入步骤不太可能发生的机理差异。揭示了解释参与双碳官能化的芳基碘化物和烯基溴化物之间区域选择性不同的主要原因。此外,在Ni催化的二碳官能化反应中使用卤化炔基可以逆转2,1-区域选择性,这是通过OA,过渡金属化,MI和RE的连续步骤完成的。与其他两种机理途径相反,合理化了OA后迁移插入步骤不太可能发生的机理差异。


























 京公网安备 11010802027423号
京公网安备 11010802027423号