当前位置:
X-MOL 学术
›
ChemPhotoChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Exploiting the Carbon and Oxa Michael Addition Reaction for the Synthesis of Yne Monomers: Towards the Conversion of Acrylates to Biocompatible Building Blocks
ChemPhotoChem ( IF 3.7 ) Pub Date : 2020-04-07 , DOI: 10.1002/cptc.201900199 Daniel Hennen 1 , Delara Hartmann 1 , Paul H. Rieger 1 , Andreas Oesterreicher 1 , Johannes Wiener 1, 2 , Florian Arbeiter 2 , Michael Feuchter 2 , Eleonore Fröhlich 3 , Margit Pichelmayer 4 , Sandra Schlögl 5 , Thomas Griesser 1
ChemPhotoChem ( IF 3.7 ) Pub Date : 2020-04-07 , DOI: 10.1002/cptc.201900199 Daniel Hennen 1 , Delara Hartmann 1 , Paul H. Rieger 1 , Andreas Oesterreicher 1 , Johannes Wiener 1, 2 , Florian Arbeiter 2 , Michael Feuchter 2 , Eleonore Fröhlich 3 , Margit Pichelmayer 4 , Sandra Schlögl 5 , Thomas Griesser 1
Affiliation
|
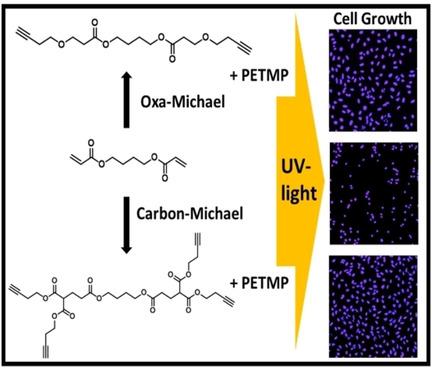
Herein, we demonstrated the synthesis of multifunctional alkyne building blocks from commercially available acrylate monomers exploiting the carbon and oxa Michael addition reaction. These compounds were obtained in decent yields and show similar or even higher photoreactivity than the initial acrylates. Importantly, selected thiol‐yne formulations can be processed by stereolithography and significantly outperform the corresponding acrylate in terms of modulus and toughness. The high compatibility of such cured materials with osteosarcoma cells makes these photopolymers interesting for hard tissue engineering.
中文翻译:

利用碳和氧杂Michael加成反应合成Yne单体:丙烯酸酯向生物相容性结构单元的转化
在本文中,我们证明了利用碳和氧杂迈克尔加成反应从市售丙烯酸酯单体合成多功能炔烃基团的方法。这些化合物以适当的收率获得,并且显示出与起始丙烯酸酯相似或什至更高的光反应性。重要的是,可以通过立体平版印刷术处理选定的硫醇炔制剂,就模量和韧性而言,它们明显优于相应的丙烯酸酯。此类固化材料与骨肉瘤细胞的高度相容性使这些光敏聚合物成为硬组织工程的有趣材料。
更新日期:2020-04-07
中文翻译:

利用碳和氧杂Michael加成反应合成Yne单体:丙烯酸酯向生物相容性结构单元的转化
在本文中,我们证明了利用碳和氧杂迈克尔加成反应从市售丙烯酸酯单体合成多功能炔烃基团的方法。这些化合物以适当的收率获得,并且显示出与起始丙烯酸酯相似或什至更高的光反应性。重要的是,可以通过立体平版印刷术处理选定的硫醇炔制剂,就模量和韧性而言,它们明显优于相应的丙烯酸酯。此类固化材料与骨肉瘤细胞的高度相容性使这些光敏聚合物成为硬组织工程的有趣材料。



























 京公网安备 11010802027423号
京公网安备 11010802027423号