PLOS Medicine ( IF 15.8 ) Pub Date : 2020-03-09 , DOI: 10.1371/journal.pmed.1003051 Elaine W Yu 1, 2 , Liu Gao 1 , Petr Stastka 1 , Michael C Cheney 1 , Jasmin Mahabamunuge 3 , Mariam Torres Soto 3 , Christopher B Ford 4 , Jessica A Bryant 4 , Matthew R Henn 4 , Elizabeth L Hohmann 2, 3
|
Background
There is intense interest about whether modulating gut microbiota can impact systemic metabolism. We investigated the safety of weekly oral fecal microbiota transplantation (FMT) capsules from healthy lean donors and their ability to alter gut microbiota and improve metabolic outcomes in patients with obesity.
Methods and findings
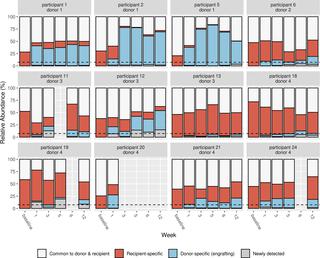
FMT-TRIM was a 12-week double-blind randomized placebo-controlled pilot trial of oral FMT capsules performed at a single US academic medical center. Between August 2016 and April 2018, we randomized 24 adults with obesity and mild–moderate insulin resistance (homeostatic model assessment of insulin resistance [HOMA-IR] between 2.0 and 8.0) to weekly healthy lean donor FMT versus placebo capsules for 6 weeks. The primary outcome, assessed by intention to treat, was change in insulin sensitivity between 0 and 6 weeks as measured by hyperinsulinemic euglycemic clamps. Additional metabolic parameters were evaluated at 0, 6, and 12 weeks, including HbA1c, body weight, body composition by dual-energy X-ray absorptiometry, and resting energy expenditure by indirect calorimetry. Fecal samples were serially collected and evaluated via 16S V4 rRNA sequencing. Our study population was 71% female, with an average baseline BMI of 38.8 ± 6.7 kg/m2 and 41.3 ± 5.1 kg/m2 in the FMT and placebo groups, respectively. There were no statistically significant improvements in insulin sensitivity in the FMT group compared to the placebo group (+5% ± 12% in FMT group versus −3% ± 32% in placebo group, mean difference 9%, 95% CI −5% to 28%, p = 0.16). There were no statistically significant differences between groups for most of the other secondary metabolic outcomes, including HOMA-IR (mean difference 0.2, 95% CI −0.9 to 0.9, p = 0.96) and body composition (lean mass mean difference −0.1 kg, 95% CI −1.9 to 1.6 kg, p = 0.87; fat mass mean difference 1.2 kg, 95% CI −0.6 to 3.0 kg, p = 0.18), over the 12-week study. We observed variable engraftment of donor bacterial groups among FMT recipients, which persisted throughout the 12-week study. There were no significant differences in adverse events (AEs) (10 versus 5, p = 0.09), and no serious AEs related to FMT. Limitations of this pilot study are the small sample size, inclusion of participants with relatively mild insulin resistance, and lack of concurrent dietary intervention.
Conclusions
Weekly administration of FMT capsules in adults with obesity results in gut microbiota engraftment in most recipients for at least 12 weeks. Despite engraftment, we did not observe clinically significant metabolic effects during the study.
Trial registration
ClinicalTrials.gov NCT02530385.
中文翻译:

粪便微生物群移植改善肥胖代谢:FMT-TRIM 双盲安慰剂对照试验。
背景
人们对调节肠道微生物群是否会影响全身代谢产生了浓厚的兴趣。我们研究了来自健康瘦肉捐赠者的每周口服粪便微生物群移植 (FMT) 胶囊的安全性,以及它们改变肠道微生物群和改善肥胖患者代谢结果的能力。
方法和结果
FMT-TRIM 是一项在美国一家学术医疗中心进行的为期 12 周的口服 FMT 胶囊双盲随机安慰剂对照试点试验。2016 年 8 月至 2018 年 4 月期间,我们将 24 名肥胖且患有轻度至中度胰岛素抵抗(胰岛素抵抗的稳态模型评估 [HOMA-IR] 在 2.0 至 8.0 之间)的成年人随机分为每周接受健康瘦捐赠者 FMT 与安慰剂胶囊组,为期 6 周。通过治疗意向评估的主要结局是通过高胰岛素正常血糖钳测量的 0 至 6 周之间胰岛素敏感性的变化。在第 0、6 和 12 周时评估其他代谢参数,包括糖化血红蛋白 (HbA1c)、体重、通过双能 X 射线吸收测定法测定的身体成分,以及通过间接热量测定法测定的静息能量消耗。通过 16S V4 rRNA 测序连续收集和评估粪便样本。我们的研究人群中 71% 为女性,平均基线 BMI 为 38.8 ± 6.7 kg/mFMT 组和安慰剂组分别为2和 41.3 ± 5.1 kg/m 2 。与安慰剂组相比,FMT 组的胰岛素敏感性没有统计学上的显着改善(FMT 组 +5% ± 12%,安慰剂组 -3% ± 32%,平均差异 9%,95% CI -5%至 28%,p = 0.16)。大多数其他次要代谢结果,包括 HOMA-IR(平均差 0.2,95% CI -0.9 至 0.9,p = 0.96 )和身体成分(去脂体重平均差 -0.1 kg, 95% CI -1.9 至 1.6 kg,p = 0.87;脂肪量平均差 1.2 kg,95% CI -0.6 至 3.0 kg,p =0.18),在为期 12 周的研究中。我们观察到 FMT 接受者中供体细菌群的植入存在差异,这种情况在整个 12 周的研究中持续存在。不良事件 (AE) 没有显着差异(10 例与 5 例,p = 0.09),并且没有与 FMT 相关的严重 AE。这项试点研究的局限性是样本量小、纳入的参与者具有相对轻微的胰岛素抵抗,并且缺乏同时的饮食干预。
结论
肥胖成人每周服用 FMT 胶囊可使大多数接受者的肠道微生物群植入至少 12 周。尽管植入,我们在研究期间没有观察到临床上显着的代谢效应。
试用注册
ClinicalTrials.gov NCT02530385。



























 京公网安备 11010802027423号
京公网安备 11010802027423号