Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Vimentin prevents a miR-dependent negative regulation of tissue factor mRNA during epithelial-mesenchymal transitions and facilitates early metastasis.
Oncogene ( IF 8 ) Pub Date : 2020-03-10 , DOI: 10.1038/s41388-020-1244-1 Marie-Emilie Francart 1 , Aline M Vanwynsberghe 1 , Justine Lambert 1 , Morgane Bourcy 1 , Anthony Genna 1 , Julien Ancel 2, 3 , Jennifer Perez-Boza 4 , Agnès Noël 1 , Philippe Birembaut 3 , Ingrid Struman 4 , Myriam Polette 3, 5 , Christine Gilles 1
Oncogene ( IF 8 ) Pub Date : 2020-03-10 , DOI: 10.1038/s41388-020-1244-1 Marie-Emilie Francart 1 , Aline M Vanwynsberghe 1 , Justine Lambert 1 , Morgane Bourcy 1 , Anthony Genna 1 , Julien Ancel 2, 3 , Jennifer Perez-Boza 4 , Agnès Noël 1 , Philippe Birembaut 3 , Ingrid Struman 4 , Myriam Polette 3, 5 , Christine Gilles 1
Affiliation
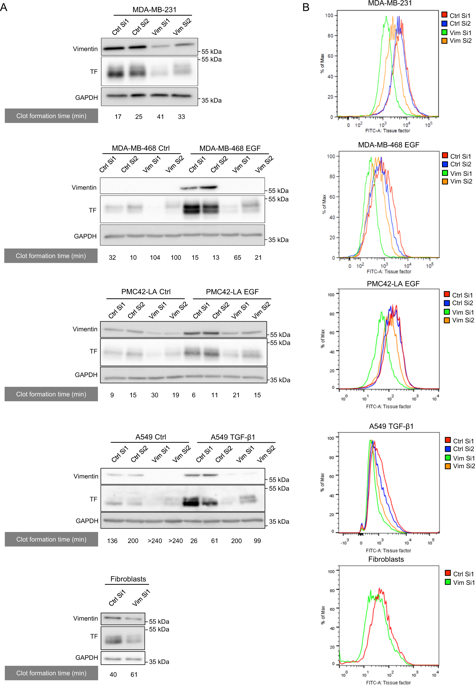
|
Epithelial-mesenchymal transitions (EMTs) are high-profile in the field of circulating tumor cells (CTCs). EMT-shifted CTCs are considered to encompass pre-metastatic subpopulations though underlying molecular mechanisms remain elusive. Our previous work identified tissue factor (TF) as an EMT-induced gene providing tumor cells with coagulant properties and supporting metastatic colonization by CTCs. We here report that vimentin, the type III intermediate filament considered a canonical EMT marker, contributes to TF regulation and positively supports coagulant properties and early metastasis. Different evidence further pointed to a new post-transcriptional regulatory mechanism of TF mRNA by vimentin: (1) vimentin silencing accelerated TF mRNA decay after actinomycin D treatment, reflecting TF mRNA stabilization, (2) RNA immunoprecipitation revealed enriched levels of TF mRNA in vimentin immunoprecipitate, (3) TF 3'-UTR-luciferase reporter vector assays implicated the 3'-UTR of TF mRNA in vimentin-dependent TF regulation, and (4) using different TF 3'UTR-luciferase reporter vectors mutated for potential miR binding sites and specific Target Site Blockers identified a key miR binding site in vimentin-dependent TF mRNA regulation. All together, these data support a novel mechanism by which vimentin interferes with a miR-dependent negative regulation of TF mRNA, thereby promoting coagulant activity and early metastasis of vimentin-expressing CTCs.
中文翻译:

波形蛋白可防止上皮-间质转化过程中组织因子 mRNA 的 miR 依赖性负调节,并促进早期转移。
上皮间质转化(EMT)在循环肿瘤细胞(CTC)领域备受瞩目。尽管潜在的分子机制仍然难以捉摸,但 EMT 转变的 CTC 被认为涵盖了转移前的亚群。我们之前的工作将组织因子 (TF) 确定为 EMT 诱导基因,为肿瘤细胞提供凝血特性并支持 CTC 的转移定植。我们在这里报告,波形蛋白(被认为是典型 EMT 标记的 III 型中间丝)有助于 TF 调节,并积极支持凝血特性和早期转移。不同的证据进一步指出了波形蛋白对 TF mRNA 的新转录后调节机制:(1)波形蛋白沉默加速了放线菌素 D 处理后 TF mRNA 的衰减,反映了 TF mRNA 的稳定性,(2) RNA 免疫沉淀揭示了波形蛋白免疫沉淀中 TF mRNA 的富集水平,(3) TF 3'-UTR-荧光素酶报告载体检测表明 TF mRNA 的 3'-UTR 参与了波形蛋白依赖性 TF 调节,以及 (4) 使用不同的方法TF 3'UTR-荧光素酶报告载体针对潜在的 miR 结合位点进行突变,并且特定的靶位点阻断剂确定了波形蛋白依赖性 TF mRNA 调节中的关键 miR 结合位点。总之,这些数据支持了一种新机制,即波形蛋白干扰 TF mRNA 的 miR 依赖性负调节,从而促进表达波形蛋白的 CTC 的凝血活性和早期转移。(4) 使用针对潜在 miR 结合位点突变的不同 TF 3'UTR-荧光素酶报告载体和特定靶位点阻断剂,鉴定了波形蛋白依赖性 TF mRNA 调节中的关键 miR 结合位点。总之,这些数据支持了一种新机制,即波形蛋白干扰 TF mRNA 的 miR 依赖性负调节,从而促进表达波形蛋白的 CTC 的凝血活性和早期转移。(4) 使用针对潜在 miR 结合位点突变的不同 TF 3'UTR-荧光素酶报告载体和特定靶位点阻断剂,鉴定了波形蛋白依赖性 TF mRNA 调节中的关键 miR 结合位点。总之,这些数据支持了一种新机制,即波形蛋白干扰 TF mRNA 的 miR 依赖性负调节,从而促进表达波形蛋白的 CTC 的凝血活性和早期转移。
更新日期:2020-03-10
中文翻译:

波形蛋白可防止上皮-间质转化过程中组织因子 mRNA 的 miR 依赖性负调节,并促进早期转移。
上皮间质转化(EMT)在循环肿瘤细胞(CTC)领域备受瞩目。尽管潜在的分子机制仍然难以捉摸,但 EMT 转变的 CTC 被认为涵盖了转移前的亚群。我们之前的工作将组织因子 (TF) 确定为 EMT 诱导基因,为肿瘤细胞提供凝血特性并支持 CTC 的转移定植。我们在这里报告,波形蛋白(被认为是典型 EMT 标记的 III 型中间丝)有助于 TF 调节,并积极支持凝血特性和早期转移。不同的证据进一步指出了波形蛋白对 TF mRNA 的新转录后调节机制:(1)波形蛋白沉默加速了放线菌素 D 处理后 TF mRNA 的衰减,反映了 TF mRNA 的稳定性,(2) RNA 免疫沉淀揭示了波形蛋白免疫沉淀中 TF mRNA 的富集水平,(3) TF 3'-UTR-荧光素酶报告载体检测表明 TF mRNA 的 3'-UTR 参与了波形蛋白依赖性 TF 调节,以及 (4) 使用不同的方法TF 3'UTR-荧光素酶报告载体针对潜在的 miR 结合位点进行突变,并且特定的靶位点阻断剂确定了波形蛋白依赖性 TF mRNA 调节中的关键 miR 结合位点。总之,这些数据支持了一种新机制,即波形蛋白干扰 TF mRNA 的 miR 依赖性负调节,从而促进表达波形蛋白的 CTC 的凝血活性和早期转移。(4) 使用针对潜在 miR 结合位点突变的不同 TF 3'UTR-荧光素酶报告载体和特定靶位点阻断剂,鉴定了波形蛋白依赖性 TF mRNA 调节中的关键 miR 结合位点。总之,这些数据支持了一种新机制,即波形蛋白干扰 TF mRNA 的 miR 依赖性负调节,从而促进表达波形蛋白的 CTC 的凝血活性和早期转移。(4) 使用针对潜在 miR 结合位点突变的不同 TF 3'UTR-荧光素酶报告载体和特定靶位点阻断剂,鉴定了波形蛋白依赖性 TF mRNA 调节中的关键 miR 结合位点。总之,这些数据支持了一种新机制,即波形蛋白干扰 TF mRNA 的 miR 依赖性负调节,从而促进表达波形蛋白的 CTC 的凝血活性和早期转移。


























 京公网安备 11010802027423号
京公网安备 11010802027423号