当前位置:
X-MOL 学术
›
Nat. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Connecting remote C-H bond functionalization and decarboxylative coupling using simple amines.
Nature Chemistry ( IF 21.8 ) Pub Date : 2020-03-09 , DOI: 10.1038/s41557-020-0428-1 Francisco de Azambuja 1, 2 , Ming-Hsiu Yang 1, 3 , Taisiia Feoktistova 4 , Manikandan Selvaraju 1 , Alexander C Brueckner 4 , Markas A Grove 4 , Suvajit Koley 1 , Paul Ha-Yeon Cheong 4 , Ryan A Altman 1
Nature Chemistry ( IF 21.8 ) Pub Date : 2020-03-09 , DOI: 10.1038/s41557-020-0428-1 Francisco de Azambuja 1, 2 , Ming-Hsiu Yang 1, 3 , Taisiia Feoktistova 4 , Manikandan Selvaraju 1 , Alexander C Brueckner 4 , Markas A Grove 4 , Suvajit Koley 1 , Paul Ha-Yeon Cheong 4 , Ryan A Altman 1
Affiliation
|
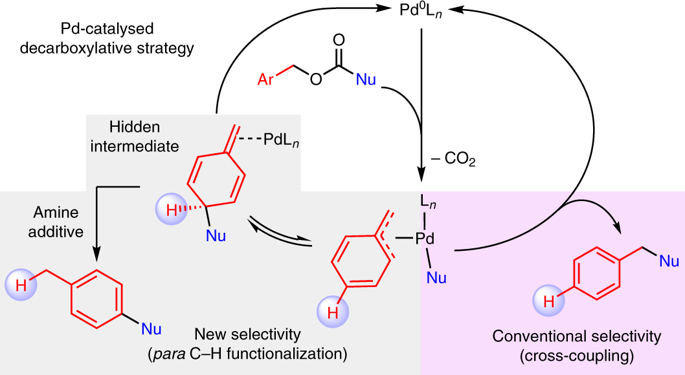
Transition metal-catalysed C-H functionalization and decarboxylative coupling are two of the most notable synthetic strategies developed in the past 30 years. Here, we connect these two reaction pathways using bases and a simple Pd-based catalyst system to promote a para-selective C-H functionalization reaction from benzylic electrophiles. Experimental and computational mechanistic studies suggest a pathway that involves an uncommon Pd-catalysed dearomatization of the benzyl moiety followed by a base-enabled rearomatization through a formal 1,5-hydrogen migration. This reaction complements 'C-H activation' strategies that convert inert C-H bonds into C-metal bonds prior to C-C bond formation. Instead, this reaction exploits an inverted sequence and promotes C-C bond formation prior to deprotonation. These studies provide an opportunity to develop general para-selective C-H functionalization reactions from benzylic electrophiles and show how new reactive modalities may be accessed with careful control of the reaction conditions.
中文翻译:

使用简单的胺连接远程CH键官能化和脱羧偶联。
过渡金属催化的CH官能化和脱羧偶联是过去30年来开发的最著名的两种合成策略。在这里,我们使用碱和简单的基于Pd的催化剂体系连接这两个反应路径,以促进由苄基亲电试剂进行的对位CH官能化反应。实验和计算机制研究表明,该途径涉及罕见的Pd催化的苄基部分脱芳香化作用,然后通过正式的1,5-氢迁移进行碱能的重新芳构化。该反应是“ CH活化”策略的补充,该策略可在CC键形成之前将惰性CH键转换为C-金属键。取而代之的是,该反应利用了反向序列并促进了去质子化之前CC键的形成。
更新日期:2020-04-24
中文翻译:

使用简单的胺连接远程CH键官能化和脱羧偶联。
过渡金属催化的CH官能化和脱羧偶联是过去30年来开发的最著名的两种合成策略。在这里,我们使用碱和简单的基于Pd的催化剂体系连接这两个反应路径,以促进由苄基亲电试剂进行的对位CH官能化反应。实验和计算机制研究表明,该途径涉及罕见的Pd催化的苄基部分脱芳香化作用,然后通过正式的1,5-氢迁移进行碱能的重新芳构化。该反应是“ CH活化”策略的补充,该策略可在CC键形成之前将惰性CH键转换为C-金属键。取而代之的是,该反应利用了反向序列并促进了去质子化之前CC键的形成。


























 京公网安备 11010802027423号
京公网安备 11010802027423号