Journal of CO2 Utilization ( IF 7.7 ) Pub Date : 2020-03-10 , DOI: 10.1016/j.jcou.2020.03.003 Hyeongbin Jeon , Monica Louise T. Triviño , Soonha Hwang , Jun Hyuk Moon , Jungho Yoo , Jeong Gil Seo
|
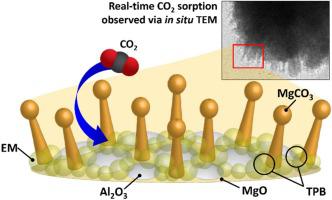
Supports are commonly used to increase the stability and prevent the agglomeration of solid CO2 sorbents. However, the exact surface changes and carbonation mechanism on supported sorbents have not been directly observed. Knowing such insight would not only allow a better understanding of the surface phenomenon on supported sorbents but would also help to identify the exact role of the supports. In this study, real-time CO2 sorption observations on Al2O3-supported eutectic mixture (EM)-promoted MgO sorbents were obtained to determine the role of Al2O3 support in the sorption process. EM-MgO-Al2O3 exhibited stable sorption-regeneration cyclic performance and maintained a capacity of ∼13 wt% after 12 cycles. In situ TEM was then utilized to directly observe the sorption and regeneration phenomenon on the sorbent. Results show that MgCO3 evolution proceeds with the formation of separated mushroom-like branches along the surface of the sorbent, contrary to the surface layer formation on unsupported sorbents. The same mushroom-like morphology was also observed during resorption, although smaller yet denser products formed due to MgO regeneration. Such morphology was observed due to the presence of Al2O3, which alters the sorbent surface, redistributes triple-phase boundaries, and directs the formation of MgCO3 towards the MgO active sites. This consequently avoids MgCO3 agglomeration and improves the cyclic stability of the sorbent. Hence, a better understanding of the carbonation mechanism and the role of support in solid sorbents was obtained.
中文翻译:

揭示熔融盐促进的MgO-Al 2 O 3吸附剂的碳化机理
载体通常用于增加稳定性并防止固体CO 2吸附剂结块。然而,还没有直接观察到载体吸附剂的确切表面变化和碳化机理。知道这种见解不仅可以更好地理解负载型吸附剂上的表面现象,还可以帮助确定载体的确切作用。在这项研究中,实时CO 2对Al吸附观测2 ö 3 -支持低共熔混合物(EM)促进的,得到的MgO的吸附剂,以确定A1的角色2 Ó 3支撑在吸附过程。EM-MgO-Al 2 O 3表现出稳定的吸附-再生循环性能,并在12个循环后保持约13 wt%的容量。然后利用原位透射电镜直接观察吸附剂上的吸附和再生现象。结果表明,MgCO 3的释放随着吸附剂表面上分离的蘑菇状分支的形成而进行,这与无载体吸附剂上的表面层形成相反。在吸收过程中也观察到相同的蘑菇状形态,尽管由于MgO再生而形成了较小但致密的产物。观察到这种形态是由于存在Al 2 O 3,Al 2 O 3改变了吸附剂表面,重新分布了三相边界,并指导了MgCO 3的形成。对MgO活动站点。因此,这避免了MgCO 3的团聚并提高了吸附剂的循环稳定性。因此,可以更好地了解碳酸化机理以及载体在固体吸附剂中的作用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号