当前位置:
X-MOL 学术
›
Food Hydrocoll.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Modification of myofibrillar protein via glycation: Physicochemical characterization, rheological behavior and solubility property
Food Hydrocolloids ( IF 10.7 ) Pub Date : 2020-08-01 , DOI: 10.1016/j.foodhyd.2020.105852 Yujuan Xu , Yuqi Zhao , Zhixi Wei , Hao Zhang , Ming Dong , Mingyuan Huang , Minyi Han , Xinglian Xu , Guanghong Zhou
Food Hydrocolloids ( IF 10.7 ) Pub Date : 2020-08-01 , DOI: 10.1016/j.foodhyd.2020.105852 Yujuan Xu , Yuqi Zhao , Zhixi Wei , Hao Zhang , Ming Dong , Mingyuan Huang , Minyi Han , Xinglian Xu , Guanghong Zhou
|
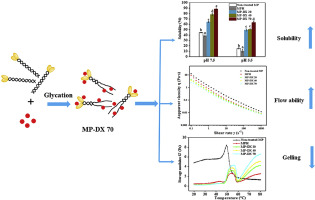
Abstract Functionalities of muscle protein are vital in the qualitative characteristics of final products. Glycation was explored as a promising way for upgrading the functionalities of muscle protein. Myofibrillar protein (MP) was conjugated with dextran (DX) in a liquid solution at a moderate temperature (37 °C). The conjugation was verified by UV–Vis spectrometry and electrophoresis analysis. The results suggested that glycation affected the particle size and morphology of MP, and glycation induced unfolding of the MP and loss of α-helix structures. As the molecular weight of DX was increased, the glycated MP displayed shear-thinning behavior with better flow ability and higher solubility, and the solubility at isoelectric point was noticeably improved (P
中文翻译:

通过糖基化修饰肌原纤维蛋白:物理化学表征、流变行为和溶解性
摘要 肌肉蛋白的功能对最终产品的定性特征至关重要。糖基化被认为是提升肌肉蛋白质功能的一种有前途的方法。肌原纤维蛋白 (MP) 在中等温度 (37 °C) 的液体溶液中与葡聚糖 (DX) 结合。通过紫外-可见分光光度法和电泳分析验证缀合。结果表明,糖化作用影响 MP 的粒径和形态,糖化作用诱导 MP 解折叠和 α-螺旋结构丢失。随着DX分子量的增加,糖化MP表现出剪切稀化行为,流动性更好,溶解度更高,等电点溶解度显着提高(P
更新日期:2020-08-01
中文翻译:

通过糖基化修饰肌原纤维蛋白:物理化学表征、流变行为和溶解性
摘要 肌肉蛋白的功能对最终产品的定性特征至关重要。糖基化被认为是提升肌肉蛋白质功能的一种有前途的方法。肌原纤维蛋白 (MP) 在中等温度 (37 °C) 的液体溶液中与葡聚糖 (DX) 结合。通过紫外-可见分光光度法和电泳分析验证缀合。结果表明,糖化作用影响 MP 的粒径和形态,糖化作用诱导 MP 解折叠和 α-螺旋结构丢失。随着DX分子量的增加,糖化MP表现出剪切稀化行为,流动性更好,溶解度更高,等电点溶解度显着提高(P


























 京公网安备 11010802027423号
京公网安备 11010802027423号