Biochimica et Biophysica Acta (BBA) - Biomembranes ( IF 3.4 ) Pub Date : 2020-03-10 , DOI: 10.1016/j.bbamem.2020.183272 Noah Kassem 1 , Maher M Kassem 2 , Stine F Pedersen 3 , Per Amstrup Pedersen 3 , Birthe B Kragelund 1
|
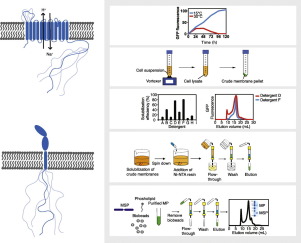
Membrane proteins exist in lipid bilayers and mediate solute transport, signal transduction, cell-cell communication and energy conversion. Their activities are fundamental for life, which make them prominent subjects of study, but access to only a limited number of high-resolution structures complicates their mechanistic understanding. The absence of such structures relates mainly to difficulties in expressing and purifying high quality membrane protein samples in large quantities. An additional layer of complexity stems from the presence of intra- and/or extra-cellular domains constituted by unstructured intrinsically disordered regions (IDR), which can be hundreds of residues long. Although IDRs form key interaction hubs that facilitate biological processes, these are regularly removed to enable structural studies. To advance mechanistic insight into intact intrinsically disordered membrane proteins, we have developed a protocol for their purification. Using engineered yeast cells for optimized expression and purification, we have purified to homogeneity two very different human membrane proteins each with >300 residues long IDRs; the sodium proton exchanger 1 and the growth hormone receptor. Subsequent to their purification we have further explored their incorporation into membrane scaffolding protein nanodiscs, which will enable future structural studies.
中文翻译:

酵母重组生产完整的人类膜蛋白,该蛋白具有较长的固有无序的细胞内区域,用于结构研究。
膜蛋白存在于脂质双层中,并介导溶质运输,信号转导,细胞间通讯和能量转换。它们的活动是生命的基础,这使它们成为重要的研究主题,但是仅能访问有限数量的高分辨率结构会使他们的机械理解复杂化。这种结构的缺乏主要涉及大量表达和纯化高质量膜蛋白样品的困难。复杂性的另一层源于由非结构性内在无序区(IDR)构成的细胞内和/或细胞外结构域的存在,该结构域可能长达数百个残基。尽管IDR形成了促进生物学过程的关键相互作用中心,但这些IDR会定期删除以进行结构研究。为了提高对完整的内在无序的膜蛋白的机制了解,我们已经开发了一种纯化方案。使用工程酵母细胞进行优化的表达和纯化,我们将两种截然不同的人膜蛋白(均具有> 300个残基的长IDR)纯化至同质。钠质子交换剂1和生长激素受体。在纯化之后,我们进一步探索了将其掺入膜支架蛋白纳米圆盘中的方法,这将有助于未来的结构研究。钠质子交换剂1和生长激素受体。在纯化之后,我们进一步探索了将其掺入膜支架蛋白纳米圆盘中的方法,这将有助于未来的结构研究。钠质子交换剂1和生长激素受体。在纯化之后,我们进一步探索了将其掺入膜支架蛋白纳米圆盘中的方法,这将有助于未来的结构研究。


























 京公网安备 11010802027423号
京公网安备 11010802027423号