Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
An Extended Conformation for K48 Ubiquitin Chains Revealed by the hRpn2:Rpn13:K48-Diubiquitin Structure.
Structure ( IF 5.7 ) Pub Date : 2020-03-10 , DOI: 10.1016/j.str.2020.02.007 Xiuxiu Lu 1 , Danielle L Ebelle 1 , Hiroshi Matsuo 2 , Kylie J Walters 1
Structure ( IF 5.7 ) Pub Date : 2020-03-10 , DOI: 10.1016/j.str.2020.02.007 Xiuxiu Lu 1 , Danielle L Ebelle 1 , Hiroshi Matsuo 2 , Kylie J Walters 1
Affiliation
|
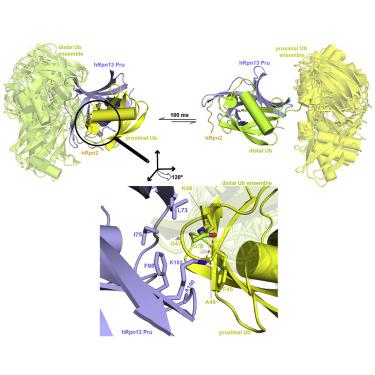
Rpn13/Adrm1 is recruited to the proteasome by PSMD1/Rpn2, where it serves as a substrate receptor that binds preferentially to K48-linked ubiquitin chains, an established signal for protein proteolysis. Here, we use NMR to solve the structure of hRpn13 Pru:hRpn2 (940-953):K48-diubiquitin. Surprisingly, hRpn2-bound hRpn13 selects a dynamic, extended conformation of K48-diubiquitin that is unique from previously determined structures. NMR experiments on free K48-diubiquitin demonstrate the presence of the reported "closed" conformation observed by crystallography, but also this more extended state, in which the hRpn13-binding surface is exposed. This extended K48-diubiquitin conformation is defined by interactions between L73 from G76-linked (distal) ubiquitin and a Y59-centered surface of K48-linked (proximal) ubiquitin. Furthermore, hRpn13 exchanges between the two ubiquitins within 100 ms, although prefers the proximal ubiquitin due to interactions with the K48 linker region. Altogether, these data lead to a revised model of how ubiquitinated substrates interact with the proteasome.
中文翻译:

hRpn2:Rpn13:K48-双泛素结构揭示的K48泛素链的扩展构象。
Rpn13 / Adrm1被PSMD1 / Rpn2募集到蛋白酶体中,在其中它作为底物受体优先与K48连接的泛素链结合,这是蛋白质蛋白水解的既定信号。在这里,我们使用NMR来解析hRpn13 Pru:hRpn2(940-953):K48-双泛素的结构。令人惊讶地,与hRpn2-结合的hRpn13选择了K48-双泛素的动态,扩展构象,该构象与先前确定的结构是独特的。游离K48-双泛素的NMR实验表明,通过晶体学观察到报告的“闭合”构象的存在,但也发现了这种更延伸的状态,其中hRpn13结合表面暴露在外。这种扩展的K48-双泛素构象是由G76连接的(远端)泛素的L73与K48连接的(近端)泛素的Y59中心表面之间的相互作用定义的。此外,hRpn13在100毫秒内在两个泛素之间交换,尽管由于与K48接头区域的相互作用而更倾向于近端泛素。总而言之,这些数据导致泛素化底物如何与蛋白酶体相互作用的修正模型。
更新日期:2020-03-10
中文翻译:

hRpn2:Rpn13:K48-双泛素结构揭示的K48泛素链的扩展构象。
Rpn13 / Adrm1被PSMD1 / Rpn2募集到蛋白酶体中,在其中它作为底物受体优先与K48连接的泛素链结合,这是蛋白质蛋白水解的既定信号。在这里,我们使用NMR来解析hRpn13 Pru:hRpn2(940-953):K48-双泛素的结构。令人惊讶地,与hRpn2-结合的hRpn13选择了K48-双泛素的动态,扩展构象,该构象与先前确定的结构是独特的。游离K48-双泛素的NMR实验表明,通过晶体学观察到报告的“闭合”构象的存在,但也发现了这种更延伸的状态,其中hRpn13结合表面暴露在外。这种扩展的K48-双泛素构象是由G76连接的(远端)泛素的L73与K48连接的(近端)泛素的Y59中心表面之间的相互作用定义的。此外,hRpn13在100毫秒内在两个泛素之间交换,尽管由于与K48接头区域的相互作用而更倾向于近端泛素。总而言之,这些数据导致泛素化底物如何与蛋白酶体相互作用的修正模型。



























 京公网安备 11010802027423号
京公网安备 11010802027423号