当前位置:
X-MOL 学术
›
Tetrahedron Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
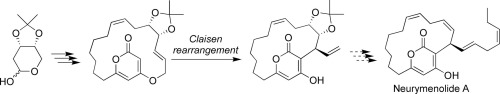
Studies toward the enantioselective synthesis of neurymenolide A: Construction of the macrocyclic core via Claisen rearrangement
Tetrahedron Letters ( IF 1.8 ) Pub Date : 2020-03-10 , DOI: 10.1016/j.tetlet.2020.151825 Masahiro Masuda , Natsuki Sakurai , Yusuke Ogura , Tetsuji Murase , Tsuneomi Kawasaki , Shohei Aiba , Naoki Mori , Hidenori Watanabe , Hirosato Takikawa
中文翻译:

神经烯内酯对映体选择性合成的研究A:通过克莱森重排构建大环核
更新日期:2020-03-10
Tetrahedron Letters ( IF 1.8 ) Pub Date : 2020-03-10 , DOI: 10.1016/j.tetlet.2020.151825 Masahiro Masuda , Natsuki Sakurai , Yusuke Ogura , Tetsuji Murase , Tsuneomi Kawasaki , Shohei Aiba , Naoki Mori , Hidenori Watanabe , Hirosato Takikawa
|
Neurymenolides, α-pyrone macrolides isolated from the Fijian red alga Neurymenia fraxinifolia, are anti-methicillin-resistant Staphylococcus aureus and anti-vancomycin-resistant Enterococcus faecium compounds. In this study, the macrocyclic core of neurymenolide A was constructed in an enantioselective manner by employing Claisen rearrangement as the key step.
中文翻译:

神经烯内酯对映体选择性合成的研究A:通过克莱森重排构建大环核
Neurymenolides,从斐济红藻Neurymenia fraxinifolia分离得到的α-吡喃酮类大环内酯,是抗甲氧西林的金黄色葡萄球菌和抗万古霉素的粪肠球菌化合物。在这项研究中,以克莱森重排为关键步骤,以对映选择性的方式构建了神经烯内酯A的大环核。


























 京公网安备 11010802027423号
京公网安备 11010802027423号