当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Aβ42 Double Mutant Inhibits Aβ42-Induced Plasma and Mitochondrial Membrane Disruption in Artificial Membranes, Isolated Organs, and Intact Cells.
ACS Chemical Neuroscience ( IF 5 ) Pub Date : 2020-03-20 , DOI: 10.1021/acschemneuro.9b00638 Ofek Oren 1 , Shani Ben Zichri 2 , Ran Taube 1 , Raz Jelinek 2 , Niv Papo 3
ACS Chemical Neuroscience ( IF 5 ) Pub Date : 2020-03-20 , DOI: 10.1021/acschemneuro.9b00638 Ofek Oren 1 , Shani Ben Zichri 2 , Ran Taube 1 , Raz Jelinek 2 , Niv Papo 3
Affiliation
|
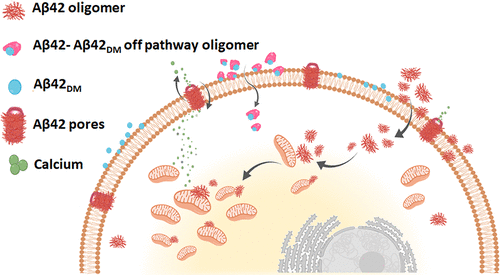
Destabilization of plasma and inner mitochondrial membranes by extra- and intracellular amyloid β peptide (Aβ42) aggregates may lead to dysregulated calcium flux through the plasma membrane, mitochondrial-mediated apoptosis, and neuronal cell death in patients with Alzheimer’s disease. In the current study, experiments performed with artificial membranes, isolated mitochondria, and neuronal cells allowed us to understand the mechanism by which a nonaggregating Aβ42 double mutant (designated Aβ42DM) exerts its neuroprotective effects. Specifically, we showed that Aβ42DM protected neuronal cells from Aβ42-induced accumulation of toxic intracellular levels of calcium and from apoptosis. Aβ42DM also inhibited Aβ42-induced mitochondrial membrane potential depolarization in the cells and abolished the Aβ42-mediated decrease in cytochrome c oxidase activity in purified mitochondrial particles. These results can be explained in terms of the amelioration by Aβ42DM of Aβ42-mediated changes in membrane fluidity in DOPC and cardiolipin/DOPC phospholipid vesicles, mimicking plasma and mitochondrial membranes, respectively. These observations are also in agreement with the inhibition by Aβ42DM of phospholipid-induced conformational changes in Aβ42 and with the fact that, unlike Aβ42, the Aβ42–Aβ42DM complex could not permeate into cells but instead remained attached to the cell membrane. Although most of the Aβ42DM molecules were localized on the cell membrane, some penetrated into the cytosol in an Aβ42-independent process, and, unlike Aβ42, did not form intracellular inclusion bodies. Overall, we provide a mechanistic explanation for the inhibitory activity of Aβ42DM against Aβ42-induced membrane permeability and cell toxicity and provide confirmatory evidence for its protective function in neuronal cells.
中文翻译:

Aβ42双突变体抑制人工膜,离体器官和完整细胞中Aβ42诱导的血浆和线粒体膜破坏。
阿尔茨海默氏病患者胞外和细胞内淀粉样β肽(Aβ42)聚集体会使血浆和线粒体内膜失稳,可能导致钙流失通过质膜,线粒体介导的细胞凋亡以及神经元细胞死亡。在目前的研究,实验的人工膜,分离的线粒体进行,神经元细胞使我们能够理解,通过该nonaggregatingAβ42双突变体(指定Aβ42的机构DM)发挥其神经保护作用。具体来说,我们发现,Aβ42 DM保护神经细胞从细胞凋亡的钙和有毒细胞内水平的Aβ42诱导积累。Aβ42 DM它还抑制细胞中Aβ42诱导的线粒体膜电位去极化,并消除了纯化的线粒体颗粒中Aβ42介导的细胞色素c氧化酶活性的降低。这些结果可在改善的方面通过解释Aβ42 DM的Aβ42介导的变化分别DOPC膜流动性和心磷脂/ DOPC磷脂囊泡,模仿血浆和线粒体膜。这些意见也与抑制Aβ42协议DM的Aβ42磷脂引起的构象变化,并与不像Aβ42,Aβ42的-Aβ42事实DM复杂不能渗透到细胞内,而是仍然附着在细胞膜。虽然大部分Aβ42的DM分子定位在细胞膜上,在不依赖Aβ42的过程中渗透到细胞质中,与Aβ42不同,它不形成细胞内包涵体。总体而言,我们提供了Aβ42的抑制活性的机理解释DM针对Aβ42诱导的膜渗透性和细胞毒性和提供一种用于在神经元细胞的保护功能验证的证据。
更新日期:2020-03-21
中文翻译:

Aβ42双突变体抑制人工膜,离体器官和完整细胞中Aβ42诱导的血浆和线粒体膜破坏。
阿尔茨海默氏病患者胞外和细胞内淀粉样β肽(Aβ42)聚集体会使血浆和线粒体内膜失稳,可能导致钙流失通过质膜,线粒体介导的细胞凋亡以及神经元细胞死亡。在目前的研究,实验的人工膜,分离的线粒体进行,神经元细胞使我们能够理解,通过该nonaggregatingAβ42双突变体(指定Aβ42的机构DM)发挥其神经保护作用。具体来说,我们发现,Aβ42 DM保护神经细胞从细胞凋亡的钙和有毒细胞内水平的Aβ42诱导积累。Aβ42 DM它还抑制细胞中Aβ42诱导的线粒体膜电位去极化,并消除了纯化的线粒体颗粒中Aβ42介导的细胞色素c氧化酶活性的降低。这些结果可在改善的方面通过解释Aβ42 DM的Aβ42介导的变化分别DOPC膜流动性和心磷脂/ DOPC磷脂囊泡,模仿血浆和线粒体膜。这些意见也与抑制Aβ42协议DM的Aβ42磷脂引起的构象变化,并与不像Aβ42,Aβ42的-Aβ42事实DM复杂不能渗透到细胞内,而是仍然附着在细胞膜。虽然大部分Aβ42的DM分子定位在细胞膜上,在不依赖Aβ42的过程中渗透到细胞质中,与Aβ42不同,它不形成细胞内包涵体。总体而言,我们提供了Aβ42的抑制活性的机理解释DM针对Aβ42诱导的膜渗透性和细胞毒性和提供一种用于在神经元细胞的保护功能验证的证据。



























 京公网安备 11010802027423号
京公网安备 11010802027423号