当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Methodology for Further Thermostabilization of an Intrinsically Thermostable Membrane Protein Using Amino Acid Mutations with Its Original Function Being Retained
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2020-03-10 , DOI: 10.1021/acs.jcim.0c00063 Satoshi Yasuda 1, 2, 3 , Tomoki Akiyama 1 , Sayaka Nemoto 1 , Tomohiko Hayashi 3 , Tetsuya Ueta 4 , Keiichi Kojima 4 , Takashi Tsukamoto 4 , Satoru Nagatoishi 5 , Kouhei Tsumoto 5, 6 , Yuki Sudo 4 , Masahiro Kinoshita 3 , Takeshi Murata 1, 2
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2020-03-10 , DOI: 10.1021/acs.jcim.0c00063 Satoshi Yasuda 1, 2, 3 , Tomoki Akiyama 1 , Sayaka Nemoto 1 , Tomohiko Hayashi 3 , Tetsuya Ueta 4 , Keiichi Kojima 4 , Takashi Tsukamoto 4 , Satoru Nagatoishi 5 , Kouhei Tsumoto 5, 6 , Yuki Sudo 4 , Masahiro Kinoshita 3 , Takeshi Murata 1, 2
Affiliation
|
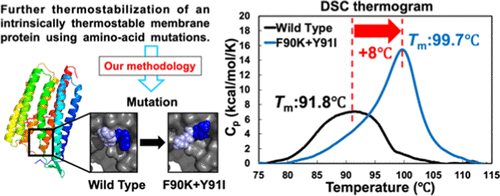
We develop a new methodology best suited to the identification of thermostabilizing mutations for an intrinsically stable membrane protein. The recently discovered thermophilic rhodopsin, whose apparent midpoint temperature of thermal denaturation Tm is measured to be ∼91.8 °C, is chosen as a paradigmatic target. In the methodology, we first regard the residues whose side chains are missing in the crystal structure of the wild type (WT) as the “residues with disordered side chains,” which make no significant contributions to the stability, unlike the other essential residues. We then undertake mutating each of the residues with disordered side chains to another residue except Ala and Pro, and the resultant mutant structure is constructed by modifying only the local structure around the mutated residue. This construction is based on the postulation that the structure formed by the other essential residues, which is nearly optimized in such a highly stable protein, should not be modified. The stability changes arising from the mutations are then evaluated using our physics-based free-energy function (FEF). We choose the mutations for which the FEF is much lower than for the WT and test them by experiments. We successfully find three mutants that are significantly more stable than the WT. A double mutant whose Tm reaches ∼100 °C is also discovered.
中文翻译:

使用固有功能保留氨基酸突变的本质上稳定的膜蛋白进一步热稳定的方法
我们开发了一种最适合识别固有稳定的膜蛋白热稳定突变的新方法。最近发现的嗜热视紫红质,其表观热变性的中点温度为T m测得的温度为〜91.8°C,被选为典型目标。在方法学中,我们首先将野生型(WT)的晶体结构中缺少侧链的残基视为“具有无序侧链的残基”,与其他基本残基不同,它们对稳定性没有重大贡献。然后,我们进行将带有无序侧链的每个残基突变为Ala和Pro以外的另一个残基,并通过仅修饰突变残基周围的局部结构来构建最终的突变体结构。这种构建是基于这样的假设,即在这种高度稳定的蛋白质中几乎已优化的其他必需残基形成的结构,不应进行修饰。然后使用我们基于物理学的自由能函数(FEF)评估突变引起的稳定性变化。我们选择FEF远低于WT的突变,并通过实验对其进行测试。我们成功地找到了三个突变体,它们比野生型稳定得多。一个双突变体,其还发现T m达到约100°C。
更新日期:2020-04-24
中文翻译:

使用固有功能保留氨基酸突变的本质上稳定的膜蛋白进一步热稳定的方法
我们开发了一种最适合识别固有稳定的膜蛋白热稳定突变的新方法。最近发现的嗜热视紫红质,其表观热变性的中点温度为T m测得的温度为〜91.8°C,被选为典型目标。在方法学中,我们首先将野生型(WT)的晶体结构中缺少侧链的残基视为“具有无序侧链的残基”,与其他基本残基不同,它们对稳定性没有重大贡献。然后,我们进行将带有无序侧链的每个残基突变为Ala和Pro以外的另一个残基,并通过仅修饰突变残基周围的局部结构来构建最终的突变体结构。这种构建是基于这样的假设,即在这种高度稳定的蛋白质中几乎已优化的其他必需残基形成的结构,不应进行修饰。然后使用我们基于物理学的自由能函数(FEF)评估突变引起的稳定性变化。我们选择FEF远低于WT的突变,并通过实验对其进行测试。我们成功地找到了三个突变体,它们比野生型稳定得多。一个双突变体,其还发现T m达到约100°C。



























 京公网安备 11010802027423号
京公网安备 11010802027423号