当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Coordination among Bond Formation/Cleavage in a Bifunctional-Catalyzed Fast Amide Hydrolysis: Evidence for an Optimized Intramolecular N-Protonation Event
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2020-03-18 , DOI: 10.1021/acs.joc.9b03383 Leandro Scorsin 1 , Ricardo F. Affeldt 1 , Bruno S. Oliveira 1 , Eduardo V. Silveira 1 , Matheus S. Ferraz 1 , Fábio P. S. de Souza 1 , Giovanni F. Caramori 1 , Fredric M. Menger 2 , Bruno S. Souza 1 , Faruk Nome 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2020-03-18 , DOI: 10.1021/acs.joc.9b03383 Leandro Scorsin 1 , Ricardo F. Affeldt 1 , Bruno S. Oliveira 1 , Eduardo V. Silveira 1 , Matheus S. Ferraz 1 , Fábio P. S. de Souza 1 , Giovanni F. Caramori 1 , Fredric M. Menger 2 , Bruno S. Souza 1 , Faruk Nome 1
Affiliation
|
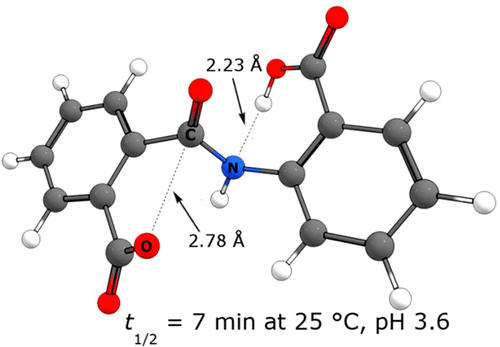
A density functional theory (DFT) computational analysis, using the ωB97X-D functional, of a rapid amide cleavage in 2-carboxyphthalanilic acid (2CPA), where the amide group is flanked by two catalytic carboxyls, reveals key mechanistic information: (a) General base catalysis by a carboxylate coupled to general acid catalysis by a carboxyl is not operative. (b) Nucleophilic attack by a carboxylate on the amide carbonyl coupled to general acid catalysis at the amide oxygen can also be ruled out. (c) A mechanistic pathway that remains viable involves general acid proton delivery to the amide nitrogen by a carboxyl, while the other carboxylate engages in nucleophilic attack upon the amide carbonyl; a substantially unchanged amide carbonyl in the transition state; two concurrent bond-forming events; and a spatiotemporal-base rate acceleration. This mechanism is supported by molecular dynamic simulations which confirm a persistent key intramolecular hydrogen bonding. These theoretical conclusions, although not easily verified by experiment, are consistent with a bell-shaped pH/rate profile but are at odds with hydrolysis mechanisms in the classic literature.
中文翻译:

双功能催化的快速酰胺水解中键形成/裂解之间的配位:分子内N质子化事件优化的证据。
使用ωB97X-D官能团对2-羧基邻苯二甲酸(2CPA)中的酰胺进行快速裂解的密度泛函理论(DFT)计算分析显示,酰胺基位于两个催化羧基的侧面,揭示了关键的机理信息:(a)羧酸盐的一般碱催化作用与羧基的一般酸催化作用不起作用。(b)也可以排除在酰胺氧上与常规酸催化偶联的酰胺羰基上羧酸盐的亲核攻击。(c)仍然可行的机制途径包括一般酸质子通过羧基传递到酰胺氮上,而另一种羧酸盐参与对酰胺羰基的亲核攻击;在过渡态的基本上未改变的酰胺羰基;两个同时发生的债券形成事件;以及时空基准速率加速。这种机制得到分子动力学模拟的支持,该分子动力学模拟确定了持久的关键分子内氢键。这些理论结论虽然不易通过实验验证,但与钟形pH /速率曲线一致,但与经典文献中的水解机理不一致。
更新日期:2020-03-19
中文翻译:

双功能催化的快速酰胺水解中键形成/裂解之间的配位:分子内N质子化事件优化的证据。
使用ωB97X-D官能团对2-羧基邻苯二甲酸(2CPA)中的酰胺进行快速裂解的密度泛函理论(DFT)计算分析显示,酰胺基位于两个催化羧基的侧面,揭示了关键的机理信息:(a)羧酸盐的一般碱催化作用与羧基的一般酸催化作用不起作用。(b)也可以排除在酰胺氧上与常规酸催化偶联的酰胺羰基上羧酸盐的亲核攻击。(c)仍然可行的机制途径包括一般酸质子通过羧基传递到酰胺氮上,而另一种羧酸盐参与对酰胺羰基的亲核攻击;在过渡态的基本上未改变的酰胺羰基;两个同时发生的债券形成事件;以及时空基准速率加速。这种机制得到分子动力学模拟的支持,该分子动力学模拟确定了持久的关键分子内氢键。这些理论结论虽然不易通过实验验证,但与钟形pH /速率曲线一致,但与经典文献中的水解机理不一致。


























 京公网安备 11010802027423号
京公网安备 11010802027423号