当前位置:
X-MOL 学术
›
ChemMedChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Solid-Phase Multicomponent Synthesis of 3-Substituted Isoindolinones Generates New Cell-Penetrating Probes as Drug Carriers.
ChemMedChem ( IF 3.4 ) Pub Date : 2020-04-02 , DOI: 10.1002/cmdc.201900656 Tlalit Massarano 1 , Alexandra Mazir 1 , Ronit Lavi 1 , Gerardo Byk 1
ChemMedChem ( IF 3.4 ) Pub Date : 2020-04-02 , DOI: 10.1002/cmdc.201900656 Tlalit Massarano 1 , Alexandra Mazir 1 , Ronit Lavi 1 , Gerardo Byk 1
Affiliation
|
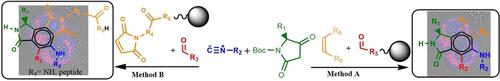
A modular solid-phase multicomponent reaction for the synthesis of 3-substituted isoindolinone derivatives has been carried out. A mixture of a chiral β-keto lactam, an aldehyde, an isocyanide and a dienophile react to produce chiral 3-substituted isoindolinones in one pot. Modularity was accomplished by using solid supported aldehydes and dienophiles. Optimization was achieved by using microwave as the source of energy. The reaction was also performed on a biologically relevant well-known programed cell death-inducing peptide D (KLAKLAK)2 on solid phase. The molecules show significant fluorescence with large Stokes shifts and fast cell penetration. The chimeric peptides can be tracked under a microscope thus proving the potential of the probes as cell sensors. They were efficiently internalized compared to unlabeled peptide, with a concomitant induction of programed cell death, thereby proving their potential as drug carriers.
中文翻译:

3-取代的异吲哚满酮的固相多组分合成产生了新的穿透细胞的探针作为药物载体。
已经进行了用于合成3-取代的异吲哚满酮衍生物的模块化固相多组分反应。手性β-酮内酰胺,醛,异氰酸酯和亲二烯体的混合物在一个锅中反应生成手性3-取代的异吲哚满酮。通过使用固体负载的醛和亲二烯体实现模块性。通过使用微波作为能源来实现优化。该反应还在固相上对生物学相关的众所周知的程序性诱导细胞死亡的肽D(KLAKLAK)2进行。分子显示出显着的荧光,具有大的斯托克斯位移和快速的细胞渗透。嵌合肽可以在显微镜下追踪,从而证明了探针作为细胞传感器的潜力。与未标记的肽相比,它们被有效地内在化了,
更新日期:2020-03-09
中文翻译:

3-取代的异吲哚满酮的固相多组分合成产生了新的穿透细胞的探针作为药物载体。
已经进行了用于合成3-取代的异吲哚满酮衍生物的模块化固相多组分反应。手性β-酮内酰胺,醛,异氰酸酯和亲二烯体的混合物在一个锅中反应生成手性3-取代的异吲哚满酮。通过使用固体负载的醛和亲二烯体实现模块性。通过使用微波作为能源来实现优化。该反应还在固相上对生物学相关的众所周知的程序性诱导细胞死亡的肽D(KLAKLAK)2进行。分子显示出显着的荧光,具有大的斯托克斯位移和快速的细胞渗透。嵌合肽可以在显微镜下追踪,从而证明了探针作为细胞传感器的潜力。与未标记的肽相比,它们被有效地内在化了,


























 京公网安备 11010802027423号
京公网安备 11010802027423号